
Science with Passion
Application No.: VBS0075
Version 1 02/2019
Group separation with Sepapure® Desalting on AZURA® Bio purification system
Ulrike Krop, Kate Monks; applications@knauer.net
KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin
Summary
Size exclusion chromatography (SEC) is a popular FPLC techniques used for protein purification. Molecules are separated according to their size. Depending on the aim of the purification high resolution fractionation or group separation is used. This application describes group separation with Sepapure Desalting and shows examples for the separation of proteins from dyes and for protein desalting.
Introduction
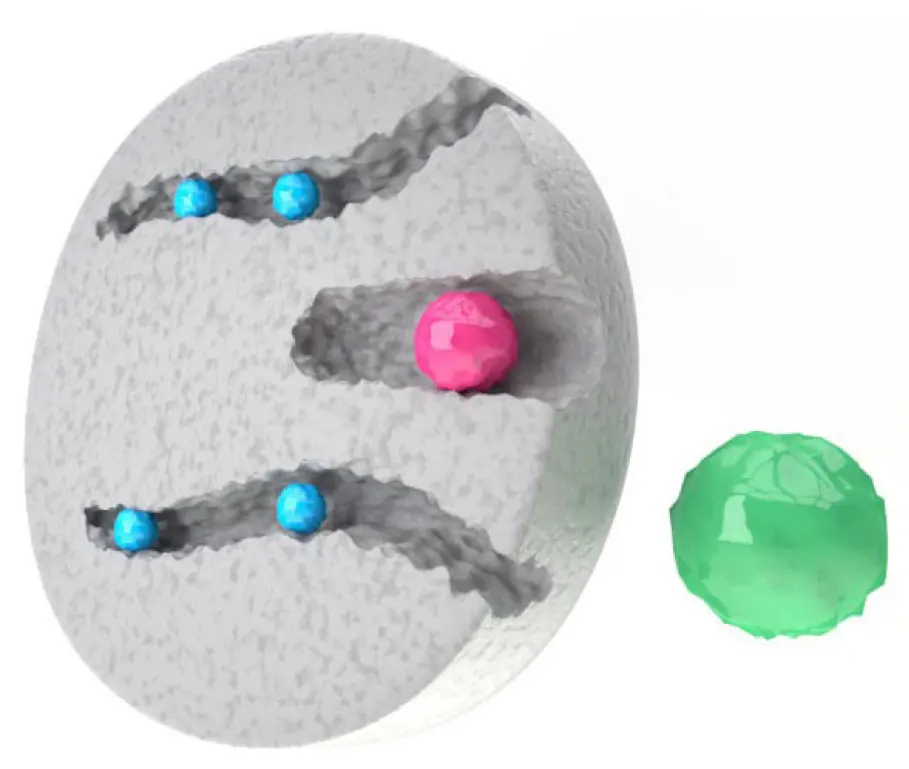
Size exclusion chromatography (SEC) separates molecules according to their different molecular size. In comparison to other chromatography methods, in SEC the sample does not interact with the column matrix. The pore size of the SEC matrix allows the distribution of molecules of different sizes over the column bed and results in separation of the sample. Bigger molecules cannot enter the pores and pass through the column eluting first from the column. The smaller the molecules the better they can enter the pores and therefore have a longer way through the column resulting in a later retention time (Fig 1). SEC can be used for high resolution fractionation or group separation of molecules. In group separation the sample is separated into two groups: the high- and low-molecular weight fraction. Group separation can be used for protein purification to remove low molecular weight contaminations like dyes or for desalting and buffer exchange. Here, Fluorescein a popular fluorescent derivatization reagent used for labeling of biomolecules was removed after the labeling process by SEC. Another common use for SEC is desalting. Proteins of interest are not retained by the column and elute first. The small salt molecules elute later and are thereby separated from the sample. This mechanism can be used as well for buffer exchange. dyes or for desalting and buffer exchange. Here, Fluorescein a popular fluorescent derivatization reagent used for labeling of biomolecules was removed after the labeling process by SEC. Another common use for SEC is desalting. Proteins of interest are not retained by the column and elute first. The small salt molecules elute later and are thereby separated from the sample. This mechanism can be used as well for buffer exchange.
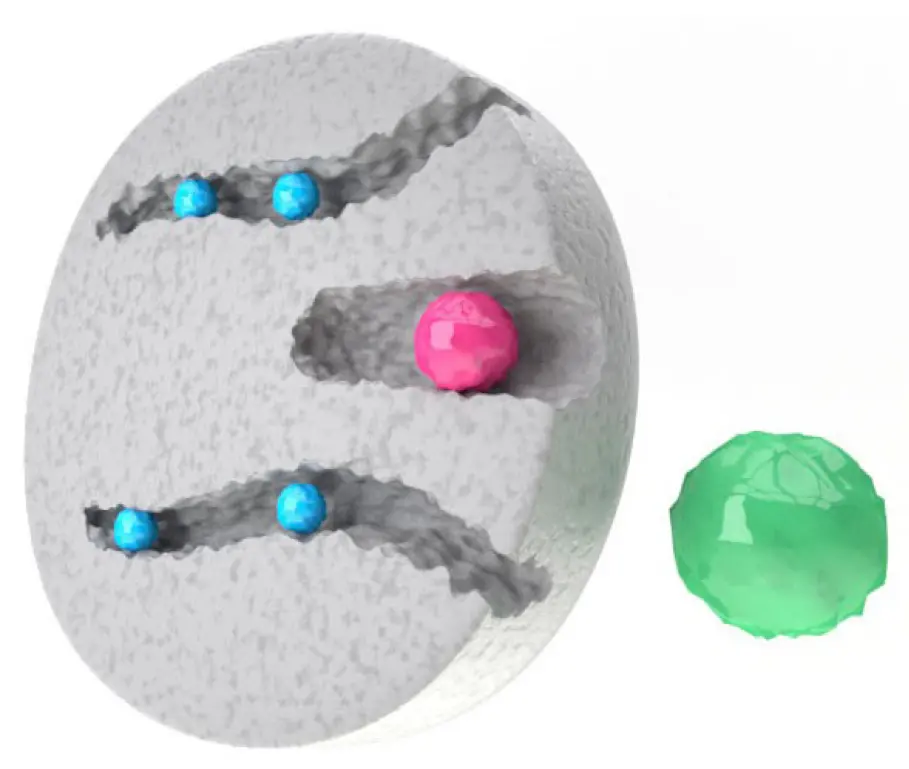
Fig. 1 Principle of size exclusion chromatography
Results
In the first method bovine Serumalbumin (BSA) was separated from 5-Carboxyfluorescein (5-FAM) (Fig 2). The high molecular weight compound BSA (Peak 1) eluted before the low molecular weight molecule 5-FAM (Peak 2) from the Desalting column. In the second method (Fig 3) BSA (Peak 1) was separated from NaCl (Peak 2).
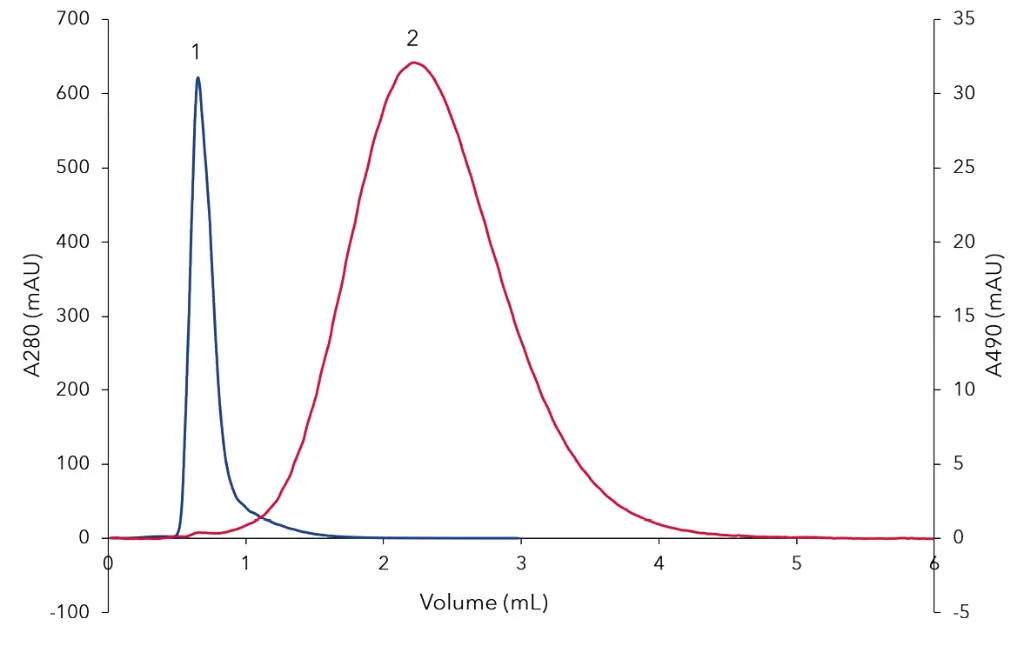
Fig. 2 Separation of BSA and 5-FAM. Peak 1 BSA, Peak 2 5-FAM, red signal UV 280nm, blue signal UV460 nm
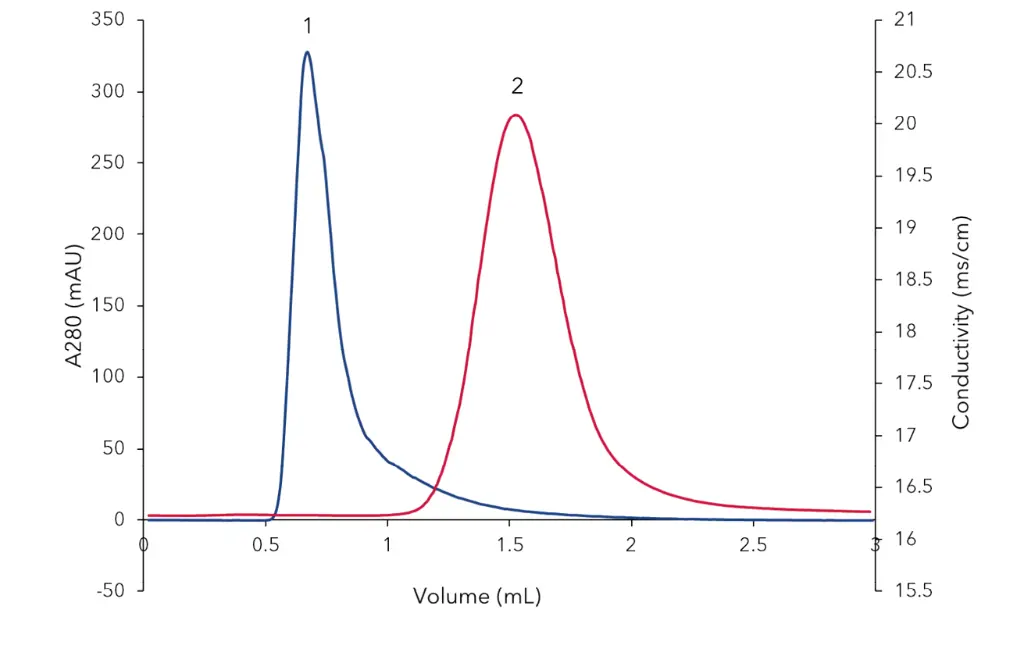
Fig. 3 Desalting of BSA. Peak 1 BSA, Peak 2 NaCl, blue signal UV280 nm,
red signal conductivity
Materials and Method
In this application, an AZURA Bio purification system consisting of AZURA P 6.1L LPG metal-free pump with 10 mL pump head; AZURA ASM 2.1L assistant module with an injection valve and a single wavelength UV detector UVD 2.1S; AZURA CM 2.1S conductivity monitor and Foxy R1 fraction collector was used. 1 mg bovine serum albumin (BSA) and 3.75 µg 5-Carboxyfluorescein (5-FAM) was dissolved in PBS. Prior to the run the 1 mL Sepapure Desalting column was equilibrated with PBS. 50 µl of the sample was injected with a flowrate of 1 mL/min. BSA was detected at 280 nm, 5-FAM was detected at 490nm and conductivity signal was recorded to monitor the salt peak.
Conclusion
Sepapure Desalting can be used for the separation of small from large molecules. BSA was separated from a fluorescent dye. Additionally, the buffer was changed by a desalting step. These two examples illustrated the principle of group separation by SEC.
Additional Materials and Methods
Tab. A1 Method parameters
Buffer A | Washing buffer: PBS (phosphate buffered saline) | ||
Gradient | isocratic | ||
Flow rate | 1 mL/min | System pressure | <3 bar |
Column temperature | RT | Run time | 6 min |
Injection volume | Each 50 µL | Injection mode | - |
Detection wavelength | 280 nm 490 nm | Data rate | 2 Hz |
Tab. A2 System configuration
Instrument | Description | Article No. |
Pump | AZURA P6.1L LPG, 10 ml PEEK | |
Assistant | AZURA ASM 2.1L Right: UVD2.1S Middle: - Left: V2.1S 6 Port/ 2Position | |
Flow cell | 3 mm semiprep, 2 µL biocompatible | |
Conductivity monitor | CM 2.1S | |
Flow cell | Preparative up to | |
Column 2 | Sepapure Desalting | |
Fraction collector | FoxyR1 | |
Software | PurityChrom, standard licence |
Related KNAUER Applications
VBS0070 – Ion Exchange Chromatography with AZURA® Bio purification system
VBS0071 – Comparison of two column sets for antibody purification in an automated two step purification process
VBS0072 – Separation of proteins with cation exchange chromatography on Sepapure SP and CM
VBS0073 – Separation of proteins with anion exchange chromatography on Sepapure Q and DEAE
VBS0074 – Comparison of Ion Exchange columns
Application details
|
Method |
FPLC |
|
Mode |
SEC |
|
Substances |
Bovine Serum Albumin (BSA), 5-Carboxyfluorescein (5-FAM) |
|
CAS number |
9048-46-8, 76823-03-5 |
|
Version |
Application No.: VBS0075 | Version 1 02/2019 | ©KNAUER Wissenschaftliche Geräte GmbH |


