Science with Passion
Application No.: VFD0185 Version 1 03/2020
Determination of biogenic amines with automated precolumn derivatization
Juliane Boettcher, Kate Monks; applications@knauer.net
KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin

Summary
Biogenic amines are found in certain foods and play a role as regulatory agents in the human metabolism [1]. In food and beverages they are formed by enzymes out of raw materials or are generated by microbiological decarboxylation of amino acids. Many of them possess a strong pharmacologic effect and some are important as precursors of hormones and components of coenzymes. The biogenic amine poisoning leads to toxicological risks and health hazards that trigger psychoactive, vasoactive, and hypertensive effects resulting from the consumption of high amounts of biogenic amines in foods [2].
Introduction
Biogenic amines are organic bases with low molecular weight. A distinction is made between aromatic amines such as histamine, tyramine, phenylethylamine and aliphatic amines like putrescine and cadaverine. They are important in a wide variety of ways. They are aroma and flavour agents; some are involved in non-enzymatic browning, whereas others are used as criteria in quality control of foodstuffs [3]. Furthermore, biogenic amines have critical biological roles in the body. They have essential physiological functions such as the regulation of growth and blood pressure or the control of the nerve conduction [2]. Histamine and tyramine are vasoactive, which means they influence decreasing or increasing blood vessel diameters. In addition, in high concentrations these substances can affect blood pressure and cause headaches, allergy-like reactions and even severe food poisoning. Putrescine and cadaverine are often cited as enhancers of these effects [3]. The following application focusses on the determination of histamine, tyramine, putrescine and cadaverine using precolumn derivatization with ortho-phthalaldehyde (OPA). The derivatization process was automated using the AZURA AS 6.1L autosampler and its “mix method” feature.
Results
First the substances were measured as single standards for identification. Then a mixed standard of tyramine, histamine, putrescine and cadaverine was used. The precolumn derivatization of the standard was performed using the mixed method option of the configured autosampler but can as well be carried out manually. Fig. 1 shows the analysis of the mixed amine standard at a concentration of 42 mg/kg for histamine, tyramine, putrescine and 64 mg/kg for cadaverine. A blank with water was measured to confirm, that the first detected peak is caused due to precolumn derivatization.
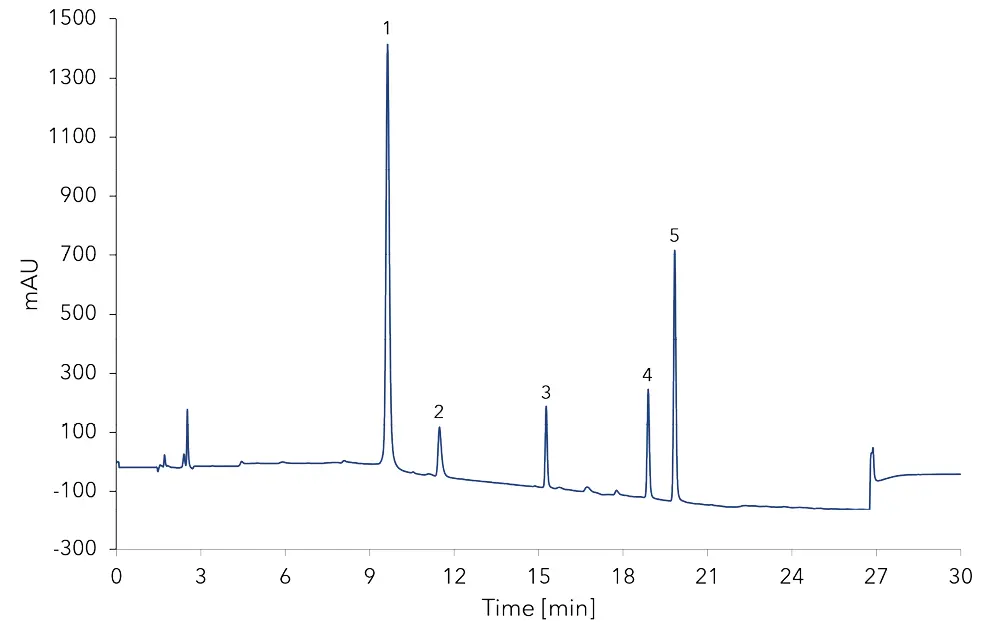
Fig. 1 Mixed amine standard.1 - derivatization peak, 2 - histamine, 3 - tyramine, 4 - putrescine, 5 - cadaverine.
Fig. 2 shows the measurement of the derivatized blank. Based on the measurement at a concentration of 10 mg/kg for histamine, tyramine, putrescine and 16 mg/kg for cadaverine, the limit of detection (LOD) and limit of quantification (LOQ) of the method was calculated.
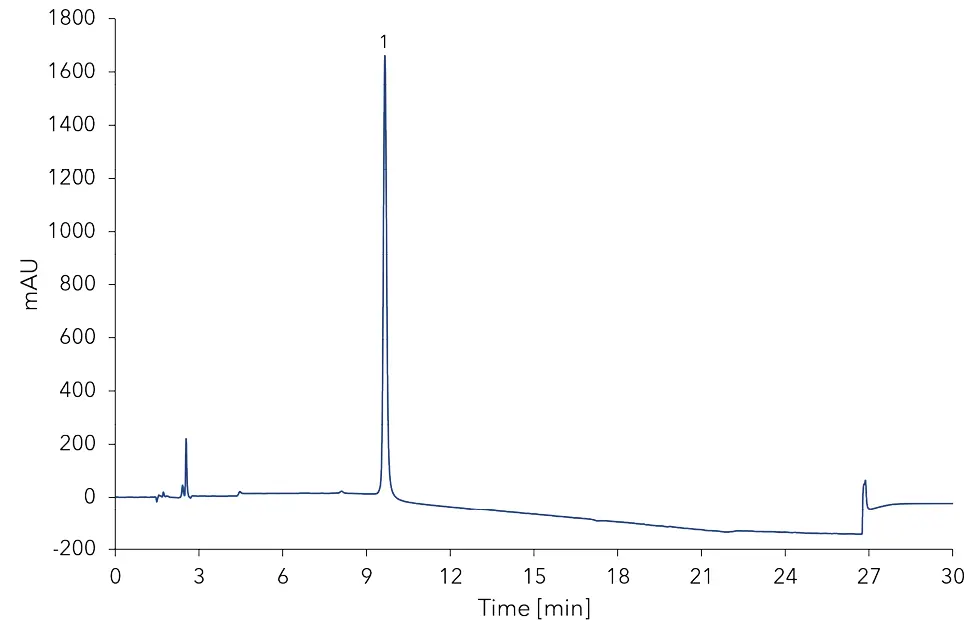
Fig. 2 Chromatogram of derivatized blank (water). 1 - derivatization peak.
Tab. 1 summarizes the calculated values for these parameters. For LOD a signal-to-noise ratio of S/N=3 was taken as basis and S/N=10 for LOQ. Furthermore, the reproducibility was checked by performing multiple measurements. Tab. 2 summarizes the relative standard deviation (%RSD) for evaluation of the parameter retention time and peak area.
Tab. 1 LOD and LOQ values for amines
LOD [mg/kg] | LOQ [mg/kg] | |
histamine | 0.28 | 0.93 |
tyramine | 0.15 | 0.50 |
putrescine | 0.13 | 0.44 |
cadaverine | 0.10 | 0.33 |
Tab. 2 Relative standard deviation for area and retention time (n=5)
Sample Preparations
All standards were dissolved in deionized water. The precolumn derivatization was performed using the “mix methods” option of the AZURA AS 6.1L autosampler. It can also be carried out manually but to ensure repeatable results the use of the autosampler is recommended. For derivatization two solutions were prepared.
Borate buffer: 0.5 M sodium tetraborate solution adjusted to pH 9.2.
OPA-reagent: 100 mg OPA were weighed in an appropriate vessel. Then add 9 mL of methanol and 1 mL of the borate buffer. After the complete dissolution of OPA, 100 µL mercaptoethanol were added.
Derivatization Protocol
Provide 350 µL of sample/standard in an autosampler vial. Add 100 µL of borate buffer (A) and mix thoroughly. Add 100 µL of the OPA reagent (B) and mix again. Wait for two minutes. Inject 1-10 µL of the derivatized sample. Fig. 3 and Fig. 4 show screenshots of the autosampler mix methods setup. In advance a position of the reagents A and B must be chosen (Fig. 4).
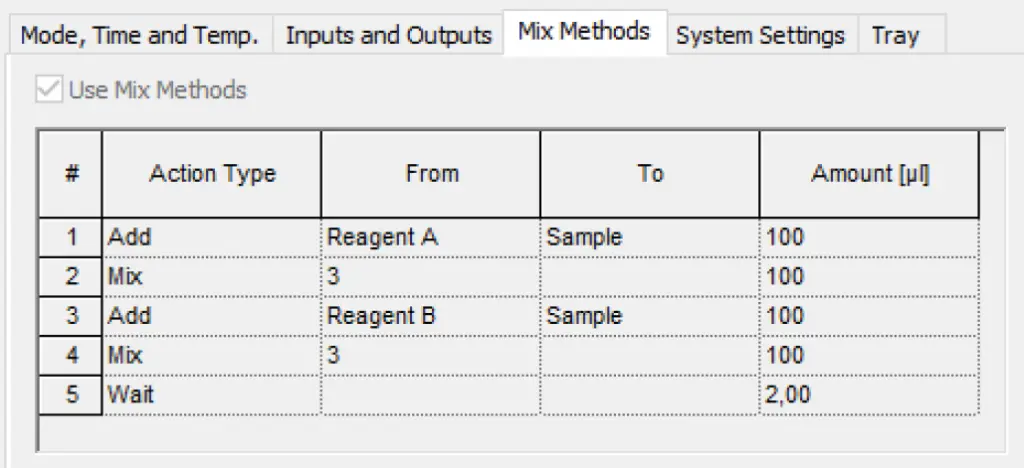
Fig. 3 Mix method setup for automated precolumn derivatization.
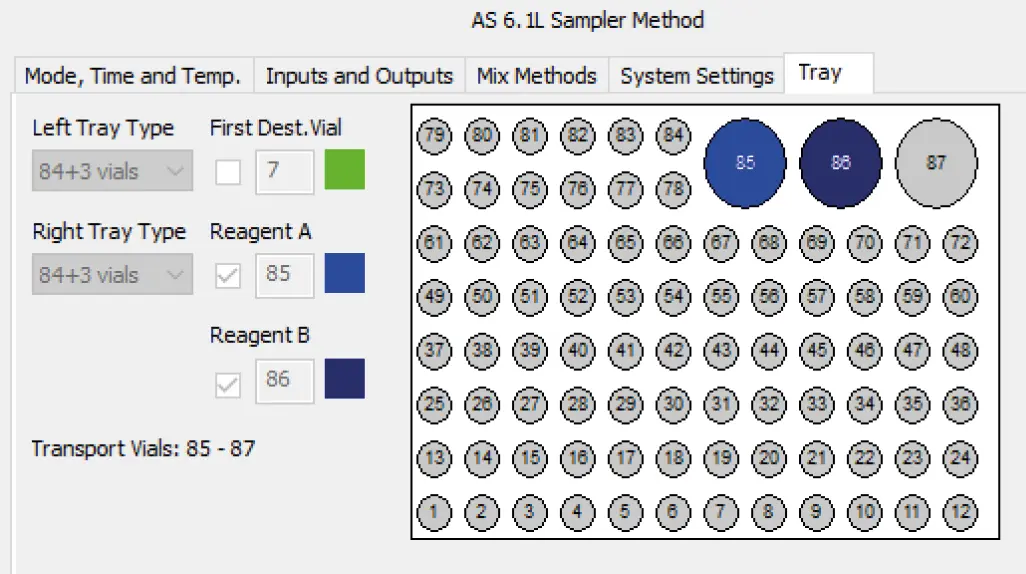
Fig. 4 Exemplarily tray positions for reagents A and B using the 84+3 autosampler tray.
Conclusion
The developed method is very robust, including the automated derivatization procedure. As seen in Tab. 2, the achieved results for area are below 1% RSD. Also, the values for retention time are reproducible and do not exceed 0.05% relative standard deviation. All peaks are baseline separated and show a resolution > 6, which is an advantage concerning disturbing matrix effects. Referring to “Commission Regulation (EU) No 1019/2013 of 23 October 2013 amending Annex I to Regulation (EC) No 2073/2005 as regards histamine in fishery products” [4] the maximum residue level (MRL) for histamine is set to 400 mg/kg. The calculated values for LOD and LOQ (0.28 mg/kg and 0.93 mg/kg) are far below MRL, which makes the applied method also suitable for food and quality control.
Materials and Methods
Tab. 3 Instrument setup
Column temperature | 25 °C | ||
Injection volume | 5 µL | ||
Injection mode | Partial Loop | ||
Detection | UV 230 nm | ||
Data rate | 20 Hz | ||
Time constant | 0.05 s | ||
Eluent (A) | 100 mM sodium acetate pH 5.8 (adjusted with acetic acid) | ||
Eluent (B) | Acetonitrile | ||
Flow rate | 1 mL/min | ||
Gradient | Time [min] | % A | % B |
0.00 | 70 | 30 | |
5.00 | 70 | 30 | |
20.00 | 20 | 80 | |
25.00 | 20 | 80 | |
25.02 | 70 | 30 | |
30.00 | 70 | 30 |
Tab. 4 System configuration
Instrument | Description | Article No. |
Pump 1 | AZURA P6.1L, LPG | |
Autosampler | AZURA AS 6.1L | |
Detector | AZURA DAD 2.1L | |
Flow Cell | Analytical KNAUER Pressure | |
Thermostat | AZURA CT 2.1 | |
Column | Eurospher 100-5 C18, 250 x 4.0 mm ID | |
Software | ClarityChrom 8.1 – Workstation, autosampler control included | |
Software | ClarityChrom 8.1 – PDA extension |

References
[1] ScienceDirect, Biogenic amine. https://www.sciencedirect.com/topics/neuroscience/biogenic-amine (January 30, 2020)
[2] Erdag, D., Merhan, O., Yildiz, B. Biochemical and pharmacological properties of biogenic amines. In: Proestos, C. Biogenic amines. IntechOpen (2018).
[3] Austrian Agency for Health and Food Safety Ltd. (AGES), Biogenic amines. https://www.ages.at/en/topics/residues-and-contaminants/biogenic-amines/ (January 30, 2020).
[4] Commission Regulation (EU) No 1019/2013 of 23 October 2013 amending Annex I to Regulation (EC) No 2073/2005 as regards histamine in fishery products Text with EEA relevance. https://eur-lex.europa.eu/eli/reg/2013/1019/oj/eng (January 30, 2020).
Related KNAUER Applications
VFD0026J – Determination of Amines in wine with precolumn dervatization (OPA)
VBS0029N – Determination of Amino acids by UHPLC with automated OPA-Derivatization by the Autosampler
Application details
|
Method |
HPLC |
|
Mode |
RP |
|
Substances |
Histamine, tyramine, putrescine, cadaverine |
|
CAS number |
51-45-6, 51-67-2, 110-60-1, 462-94-2 |
|
Version |
Application No.: VFD0185 | Version 1 03/2020 | ©KNAUER Wissenschaftliche Geräte GmbH |