
Science with Passion
Application No.: VEV0084
Version 1 10/2021
Analysis of Poly [(R)-3-hydroxybutyric acid] in chloroform using GPC and universal calibration
Nils Lüttgert, Lisa Loxterkamp, Juliane Böttcher, Kate Monks; applications@knauer.net
KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin
![Analysis of Poly [(R)-3-hydroxybutyric acid] in chloroform using GPC and universal calibration Analysis of Poly [(R)-3-hydroxybutyric acid] in chloroform using GPC and universal calibration](/web/image/186020-71f159f5/Fig-0.webp)
Photo: pixabay
Summary
Two Poly [(R)-3-hydroxybutyric acid] (PHB) standards (10 - and 500 kDa) were analysed in chloroform with SEC using universal calibration. For this purpose, a KNAUER system was used in combination with a linear GPC column. For the determination of the number average molecular mass (Mn) and the mass average molecular mass (Mw) as well as the poly dispersity (PD), polystyrene (PS) and polymethylmethacrylate (PMMA) calibrations were performed in chloroform. It could be shown that this system can be successfully used for the analysis of the low molecular weight standard. The molar mass of the 500 kDa standard, on the other hand, was greatly underestimated with this method, but this is probably related to the poor solubility or the sample preparation of the higher molecular weight standard.
Introduction
Poly [(R)-3-hydroxybutyric acid] (PHB) is the best-known representative of poly(hydroxy)alkanoates. It is a biodegradable polyester that can be produced biologically using bacteria.(1) Its physical properties are similar to polypropylene, and it is therefore used in the packaging industry as well as for medical applications.(2) In 2018, more than 30 million tons of polypropylene were processed only in the packaging industry alone.(3) This shows the magnitude of the dimension in which PHB could be used as a sustainable substitute for petrochemical plastics. The properties of manufactured plastics such as PHB are largely determined by the number average molecular mass (Mn), the weight average molecular weight (Mw) and the PDI. These parameters can be easily determined using gel permeation chromatography (GPC). In this application note a method for the analysis of PHB in chloroform by GPC using universal calibration is provided.
Sample Preparation
For calibration with polystyrene (PS), an EasiCal PS-1/2-Kit in the molar mass range 3 670 Da–2 561 kDa from Agilent was used (16-point). The calibration standards were dissolved in 1 ml chloroform for one hour at room temperature. For calibration with polymethyl methacrylate (PMMA), a ReadyCal-Kit in the molar mass range 2 380 Da–2 200 kDa from PSS was used (12-point). The calibration standards were dissolved in 1 ml chloroform for one hour at room temperature. The 10 kDa PHB standard was dissolved in chloroform over night at room temperature without stirring to a concentration of 1 mg/ml, then filtered with a 0.45 µm PTFE filter. The 500 kDa PHB standard was partially dissolved in chloroform at 60 °C for two hours with a target concentration of 1 mg/ml, then filtered with a 0.45 µm PTFE filter and analysed at 25 °C. In all cases butylated hydroxytoluene (BHT) was used as a flow marker with a concentration of 1.5 mg/ml.
Results
For this application, a linear column (300x8 mm ID) with poly(styrene-co-divinylbenzene) as the stationary phase was used. Linear columns can save costs and lower the solvent consumption compared to a commonly used combination of three columns with single porosities. With this column two universal calibrations with PS and PMMA were created (Fig. 1+2) using chloroform as the mobile phase. According to the literature, for PS the Mark-Houwink parameters K=7.86 · 105 dl/g, α=0.76 (4) and for PMMA K=4.8 · 105 dl/g, α=0.8(5) were used. For PHB the Mark-Houwink parameter K=7.7 · 105 dl/g and α=0.82 were taken as basis for calculation.(6) Fig. 3 shows the chromatogram of the 10 kDa PHB standard, which elutes after 10.9 minutes. At 12.7 min, the flow marker signal (BHT) can be observed. The PHB-standard certificate indicates an Mn value of 8 592 Da, determined by NMR. The evaluation using PS-calibration resulted in Mn= 7 632 Da (-11 % compared to the certificate). Evaluation with the PMMA calibration resulted in Mn=7 114 Da (-17 %). For the 500 kDa PHB polymer, an Mn of 153 796 Da (-69 %) (mean value from double determination) was determined by means of PS calibration and an Mn of 131 694 Da (-74 %) (mean value from double determination) by means of PMMA calibration. The chromatogram of the 500 kDa PHB Standard is shown in Fig. 4.
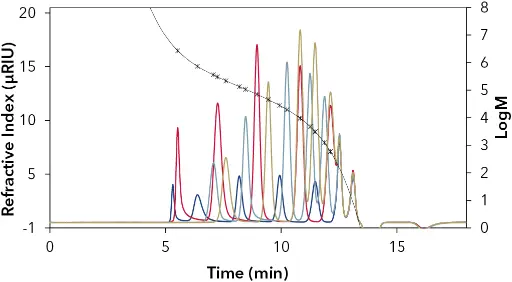
Fig. 1 16-point PS calibration in chloroform by Agilent (EasiCal). Blue=PS-1(A); Red=PS-1(B); Light blue=PS-2(A); Gold=PS-2(B). Fit function of the 5th degree with deviations less than 5 %. R2=1

Fig. 2 12-point PMMA calibration in chloroform by PSS (ReadyCal). Blue=green cup; Red=red cup; Light blue=white cup. Fit function of the 5th degree with deviations less than 5 %. R2=1

Fig. 3 Chromatogram of the 10 kDa PHB polymer. The concentration was 1 mg/ml with an injection volume of 20 µl
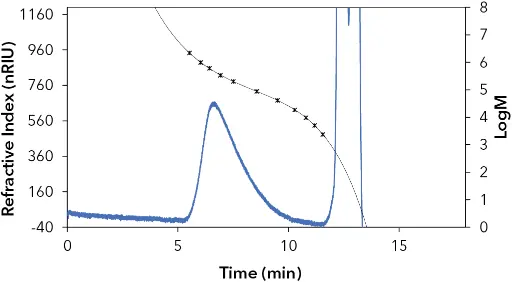
Fig. 4 Chromatogram of the 500 kDa PHB polymer. The concentration was below 1 mg/ml because the polymer was not completely dissolved
Conclusion
The low deviation of the 10 kDa PHB standard with 11 % (PS-calibration) and 17 % (PMMA calibration), show that both calibrations can be used for the determination of low molecular weight PHB. Contrary for high molecular weight PHB (500 kDa), the deviations of both calibrations are significantly too high that they cannot be used for an appropriate determination of the molecular weight. One possible explanation for this deviation would be poor solubility in chloroform due to high crystallinity of PHB. While the 10 kDa PHB polymer dissolved at room temperature, the 500 kDa polymer only dissolved partially in chloroform after 2 h at 60 °C. It is conceivable that only the low molecular weight fraction has dissolved, so that the Mn value is underestimated. The Mn determination would probably be more accurate if the sample was not cooled back to room temperature after dissolving, so that a possible crystallization of the higher molecular weight fraction would be avoided. nevertheless, low molecular weight PHB is also interesting in its application. For example, in the field of drug delivery by means of prolonged-release tablets, in which PHB is pressed with the active ingredient. The retardation time increases exponentially with decreasing molecular weight (3–60 kDa) of the PHB in the example with Midrodin·HCl.(7)
Materials and Methods
Column temperature | 25 °C |
Injection volume | 20 µL |
Injection mode | Partial Loop |
Detection | RID |
Data rate | 10 Hz |
Pump parameters | |
Eluent (A) | Chloroform |
Flow rate | 1 ml/min |
Pump program | isocratic |
References
[1] Wang, J., Bakken, L. Screening of Soil Bacteria for Poly-β-Hydroxybutyric Acid Production and Its Role in the Survival of Starvation. Microb Ecol 35, 94–101 (1998).
[2] Utsunomia C, Ren Q and Zinn M Poly(4-Hydroxybutyrate): Current State and Perspectives. Front. Bioeng. Biotechnol. 2020, 8:257.
[3] Marktstudie Polypropylene – Ceresana, 5. 2019.
[4] K. K. Chee, Polymer, 1987, 28,978.
[5] J. Bischoff and V. Desreux, Bull. Soc. Chim. Berlg. 1952, 61, 10.
[6] Marchessault RH, Okamura K, Su CJ Macromolecules. 1970, 3:735–740.
[7] Trathnigg B, Weidmann V, Lafferty RM, Korsatko B, Korsatko W: 1988. Angew Makromol Chern 161: 1.
Related KNAUER Applications
VCH0017 – Molecular weight distribution of a broad polystyrene standard
VTN0021 – Green SEC/GPC – comparison of two calibrations in three different solvents
System configuration
Instrument | Description | Article No. |
Pump | AZURA P6.1L HPG | |
Autosampler | AZURA AS 6.1L | |
Detector 1 | AZURA RID 2.1L | |
Thermostat | AZURA CT 2.1 | |
Pre-Column (CHCl3) | AppliChrom ABOA StyDiViBe 10E5A-BPT,500–1.5 MioDa, 50x8 mm, CHCl3, 100.000 A | |
Column (CHCl3) | AppliChrom ABOA StyDiViBe 10E5A-BPT,500–1.5 MioDa, 300x8 mm, CHCl3, 100.000 A | |
Software | ClarityChrom 8.2.3 – Workstation, autosampler control included | |
Software | ClarityChrom 8.2.3 – SEC/GP extension |

Application details
Method | HPLC |
Mode | GPC |
Substances | Poly [(R)-3-hydroxybutyric acid] |
CAS number | 26063-00-3 |
Version | Application No.: VEV0084 | Version 1 10/2021 | ©KNAUER Wissenschaftliche Geräte GmbH |


![Analysis of Poly [(R)-3-hydroxybutyric acid] in chloroform using GPC and universal calibration Analysis of Poly [(R)-3-hydroxybutyric acid] in chloroform using GPC and universal calibration](/web/image/186024-842c8c72/VEV0084_knauer_Analysis%2520of%2520PHB%2520in%2520Chloroform%2520using%2520GPC%2520and%2520universal%2520calibration_LR.svg)