
Science with Passion
Application No.: VBS0076
Version 1 01/2019
Purification of p-coumaric acid esterase by immobilized metal ion affinity chromatography
Mirjam Thora Sommer2, Ulrike Krop1, Jessica Thiesing-Paul1, Kate Monks1, David Thiesing2; applications@knauer.net
1KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin
2SternEnzym GmbH & Co. KG, Ahrensburg, Germany
Summary
The p-coumaric acid esterase (p-CAE) has a wide range of applications and is therefore an enzyme of interest. p-CAE was produced by Pichia pastoris and purified by immobilized metal ion affinity chromatography (IMAC). Different IMAC systems were tested and compared. 35.4% of the total protein was identified as active p-CAE. A total activity loss of 21.4% was recorded. The p-CAE could be successfully purified.
Introduction
The structure of the plant cell wall consists of covalent cross-linked polysaccharides which provide stability [1]. Hydroxycinnamic acids are not freely present in plants but can be cleaved by substrate-specific enzymes from the complex structure. One common hydroxycinnamic acid is p-coumaric acid (p-CA) [2]. The p-coumaric acid esterase (p-CAE) is known for its very high p-CA activity. It hydrolyzes the ester linkages between the polysaccharides and various hydroxycinnamic acids [3]. The p-CAE is particularly attractive for the industry due to its broad substrate specificity [4]. The p-CAE can be used in many ways in the food and pharmaceutical industries, as well as in the area of renewable resources, e. g. bioethanol production. A 6xHis-Tag was attached to the p-CAE which was recombinantly expressed in Pichia pastoris and purified by immobilized metal ion affinity chromatography (IMAC) promising better handling and a higher yield compared to the classical production.
Results
p-CAE was previously purified by IMAC with a three-step elution [4], resulting in our case in a broad elution. Thus, four different IMAC systems were further tested. Two different materials Ni-IDA and Ni-NTA and sodium phosphate buffer pH 8 and pH 6 were used. For qualitative evaluation, Fig. 1 shows the chromatograms of the elution peaks and corresponding SDS-PAGE bands. The SDS-PAGE image shows the eluate and the enzyme treated with Endo H. All impurities were separated qualitatively equal. The recovery rate of the activity and the protein content for each system are shown in Fig. 2. The largest proportion of the total activity can be found in the eluate. The total protein concentration used is entirely recovered in the pH 6 systems. Due to an additional buffering in the conditions favored for activity measurement, namely pH 8, a loss of the total protein concentration was detected. The four systems are similar in terms of recovery rates. For this reason, the system with the largest elution peak (IDA system pH 8) is selected as the optimal system. The results of this purification are summarized in Tab. 1 and the chromatogram and SDS-PAGE of all fractions are shown in Fig. 3.
Tab. 1 Purification results of p-CAE by IMAC IDA pH 8 system
Protein content (%) | Total activity (%) |
35.4 + 0.1 | 78.6 + 1.0 |
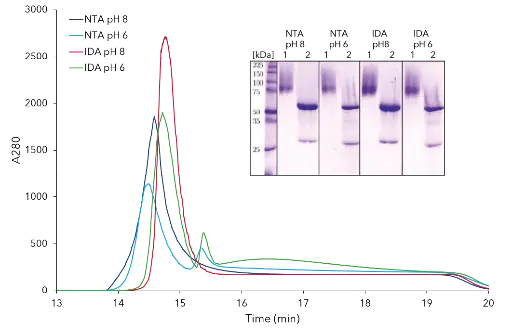
Fig. 1 Elution chromatograms at 280 nm of four IMAC systems and SDS PAGE of eluate (1) and eluate treated with Endo H (2)
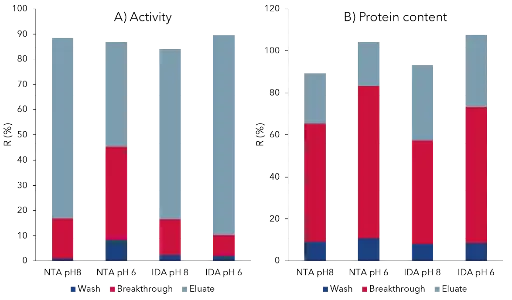
Fig. 2 Validation of four IMAC systems by recovery rate for enzyme activity and protein content
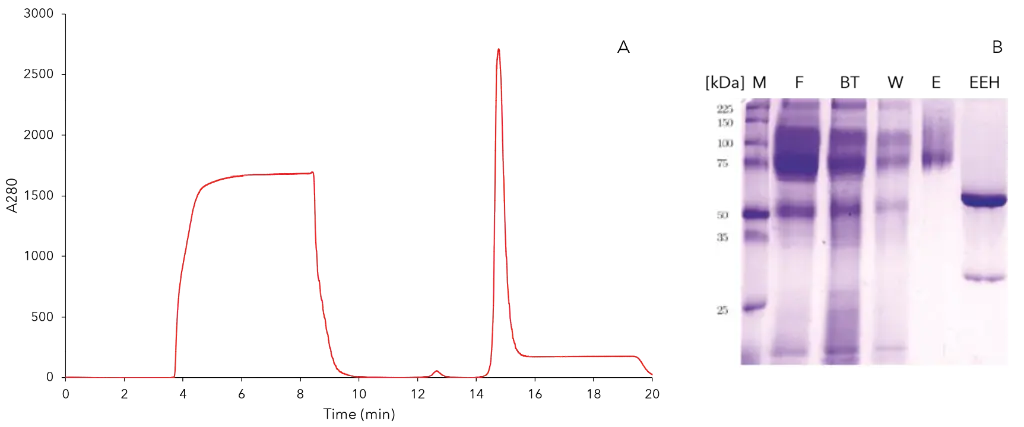
Fig. 3 Optimal IMAC system with IDA and pH 8 for purification of p-CAE; A) Chromatogramm 280nm, B) SDS page (M - marker, F - filtrate, BT - breakthrough, W - wash, E - eluat, EEH - eluat treated with Endo H)
Materials and Method
The bioinert AZURA® system consisted of a 50 mL high pressure gradient pump, a single wavelength detector, an injection valve, a 50 mL sample pump and a fraction collector. Two different 1mL IMAC columns, IDA and NTA, columns were used for the purification after immobilization with nickel ions. In addition, a comparison of two different buffers (sodium phosphate buffer pH 6 and pH 8) was made. All measurements were performed in duplicate. Bradford Assay was used to determine the total protein concentration. The activity determination of the p-CAE was carried out using an optimized enzyme assay in combination with a reverse phase HPLC method [4].
Conclusion
For the purification of p-CAE, two column materials and two pH values were tested. Comparing the Ni-IDA with the Ni-NTA column, the absorption using IDA is higher and delayed. IDA can adsorb larger amounts of protein then NTA [5]. The IDA systems are in this case beneficial. Evaluation of two different pHs shows that, the target protein elutes as one sharp peak from the column when adjusting pH to 8. At pH 6, a second smaller elution peak can be seen, which was identified also as pure target protein. IMAC should be carried out at neutral to slightly basic pH values such as pH 8, to make sure the histidyl residues of imidazole are not protonated during adsorption [6]. The slightly acidic pH system and the NTA column material were not optimal for p-CAE purification. These results led to the final procedure where p-CAE was purified with Ni-IDA column at pH 8.
References
[1] G. Williamson, P. A. Kroon, and C. B. Faulds. Hairy plant polysaccharides: a close shave with microbial esterases. Microbiology (Reading, England), 144 ( Pt 8):2011– 2023, 1998. ISSN 1350-0872. doi: 10.1099/00221287-144-8-2011.
[2] M. N. Clifford. Chlorogenic acids and other cinnamates – nature, occurrence, dietary burden, absorption and metabolism. Journal of the Science of Food and Agriculture, 80(7):1033–1043, 2000. ISSN 1097-0010.
[3] P. A. Kroon, M. T. Garcia-Conesa, I. J. Fillingham, G. P. Hazlewood, and G. Williamson. Release of ferulic acid dehydrodimers from plant cell walls by feruloyl esterases. Journal of the Science of Food and Agriculture, 79(3):428–434, 1999. ISSN 1097-0010. doi: 10.1002/(SICI)1097-0010(19990301)79:3 428:AID-JSFA2753.0.CO;2-J.
[4] A. Nieter, S. Kelle, D. Linke, and R. G. Berger. A p-coumaroyl esterase from rhizoctonia solani with a pronounced chlorogenic acid esterase activity. New Biotechnology, 37: 153–161, 2017. ISSN 1871-6784. doi: 10.1016/j.nbt.2017.01.002
[5] H. Block, B. Maertens, A. Spriestersbach, N. Brinker, J. Kubicek, R. Fabis, J. Labahn, and F. Schafer. Chapter 27: Immobilized-metal affinity chromatography. In R. R. Burgess and M. P. Deutscher, editors, Guide to protein purification, volume 463 of Methods in Enzymology, pages 39–473. Elsevier/Academic Press, Amsterdam, 2009. ISBN 9780123745361. doi: 10.1016/S0076-6879(09)63027-5.
[6] V. Gaberc-Porekar and V. Menart. Perspectives of immobilized-metal affinity chromatography. Journal of Biochemical and Biophysical Methods, 49(1-3):335–360, 2001. ISSN 0165022X. doi: 10.1016/S0165-022X(01)00207-X.
Additional Materials and Methods
Tab. A2 Method parameters
Column temperature | RT |
Injection volume | 5 mL |
Injection mode | Sample loop |
Detection wavelength | UV 280 nm |
Data rate | 2 Hz |
Tab. A3 Pump parameters (program 1)
Eluent A | 20mM Sodium phosphate buffer pH6 + 5mM imidazole + 300 mM NaCl | |
Eluent B | 20mM Sodium phosphate buffer pH6 + 250mM imidazole + 300 mM NaCl | |
Flow rate | 1 mL/min | |
Pump program | [%] A | [%] B |
0 | 100 | 0 |
3 | 100 | 0 |
13 | 100 | 0 |
13.1 | 0 | 100 |
17 | 0 | 100 |
17.1 | 100 | 0 |
20 | 100 | 0 |
Tab. A4 Pump parameters (program 2)
Eluent A | 50 mM Sodium phosphate buffer pH8 + 5 mM imidazole + 300 mM NaCl | |
Eluent B | 50 mM Sodium phosphate buffer pH8 + 250mM imidazole + 300 mM NaCl | |
Flow rate | 1 mL/min | |
Pump program | [%] A | [%] B |
0 | 100 | 0 |
3 | 100 | 0 |
13 | 100 | 0 |
13.1 | 0 | 100 |
17 | 0 | 100 |
17.1 | 100 | 0 |
20 | 100 | 0 |
Tab. A5 System configuration
Instrument | Description | Article No. |
Pump | AZURA P 6.1L High Pressure Pump with | |
Assistant | AZURA ASM 2.1L Left: UVD2.1S single wavelength detector Middle: 6Port2Pos, 1/16“, PEEK Right: P4.1L pump with 10 ml pump head | |
Conductivity monitor | AZURA CM 2.1S with flow cell - up to | |
Fraction collector | Foxy R1 | |
Column switching valve | Bioinert Multifunction Selection Valve | |
Column | NTA HisTrapTMFF Crude 1ml IDA HiTrapTM 1ml | |
Software | PurityChrom5 Basic |
Related KNAUER Applications
VBS0063 – Automated two - step purification of mouse antibody IgG1 with AZURA Bio purification system
VBS0064 – Comparison of IgG purification by two different protein A media
VBS0065 – Separation of two model proteins with ion exchange chromatography
VBS0067 – Automated two-step purification of 6xHis-tagged GFP with AZURA Bio purification system
VBS0068 – Fast and robust purification of antibodies from human serum with a new monolithic protein A column
VBS0069 – Purification of Sulfhydryl Oxidase
VBS0070 – Ion Exchange Chromatography with AZURA® Bio purification system
VBS0071 – Comparison of two column sets for antibody purification in an automated two step purification process
VBS0072 – Separation of proteins with cation exchange chromatography on Sepapure SP and CM
VBS0073 – Separation of proteins with anion exchange chromatography on Sepapure Q and DEAE
VBS0074 – Comparison of ion exchange columns
Application details
|
Method |
FPLC |
|
Mode |
Affinity |
|
Substances |
p-cumaric acid esterase |
|
CAS number |
n/a |
|
Version |
Application No.: VBS0076 | Version 1 01/2019 | ©KNAUER Wissenschaftliche Geräte GmbH |


