
Science with Passion
Application No.: VTN0023 Version 1 06/2023
Investigation of system performance – of the KNAUER AZURA® UHPLC system
Juliane Kramer, Christian Schmidt, Giorgia Greco; kramer@knauer.net
KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin

Rendering: KNAUER
Summary
Although time is relative, the total run time of a method is an important factor when it comes to high throughput analysis. Saving time means saving money. With the exemplary separation of compounds that are present in anti-epileptic drugs, the KNAUER AZURA UHPLC system equipped with the new binary AZURA P 8.1L pump, thanks to the low dispersion and operating pressure up to 1240 bar, allows to speed up your method and increase throughput preserving resolution.
Introduction
Short methods that provide good resolution are highly demanded. The reduction of analysis time per sample can be realized adjusting different method parameters, e.g., increase of temperature, optimizing the gradient elution, or change to a smaller column. Increasing the flow rate is probably the first approach users think about. But higher flow rates will also result in a higher backpressure. The KNAUER AZURA UHPLC system equipped with the new binary AZURA P 8.1L pump can be operated up to 1240 bar, which allows a broad variety of usable flow rates. At the same time, system dispersion of this high-end system is optimized to prevent band-broadening and to get the best out of your separation regarding plate counts or peak capacity. With the exemplary separation of compounds, used as active ingredients in the formulation of common anti-epileptic drugs, the effect of increased flow rate was investigated. Four different flow rates were applied in a range from 0.2 ml/min up to 1.5 ml/min. Several parameters like the retention time stability, the efficiency, and the change in peak symmetry, as indicator for the system dispersion, are discussed.
Sample Preparation
Single standards were weighed in volumetric flasks and filled up to the mark with acetonitrile:water 15:85 (v/v). The individual stock solutions had a concentration of β= 1 mg/ml. Out of these stock solutions a mixed standard was prepared and filled up with acetonitrile:water 15:85 (v/v). The used mixed standard had a concentration of β=0.01 mg/ml for each compound. 2 µl of the mixed standard were injected.
Five compounds that can be present in anti-epileptic drugs were separated: 2-ehtyl-2-phenylmalonamide (PEMA), ethosuximide, primidone, carbamazepine, and phenytoin. Additionally, caffeine was used as a reference substance. The separation at four different flow rates was investigated (0.2, 0.6, 1.2, 1.5 ml/min), and 10 replicates of the mixed standard were measured for each flow rate. The following figures show exemplary chromatograms for 0.2 and 1.5 ml/min, as well as an overlay of all flow rates to visualize the reduction in total analysis run time.
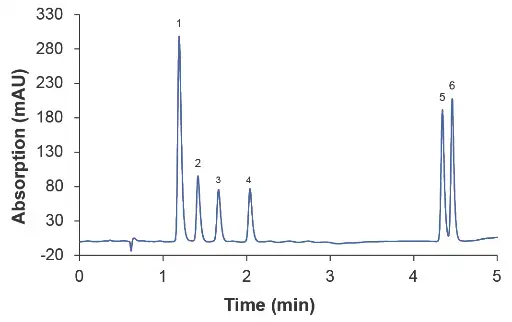
Fig. 1 Chromatogram at 0.2 ml/min, (1) caffeine, (2) PEMA, (3) ethosuximide, (4) primidone, (5) carbamazepine, (6) phenytoin
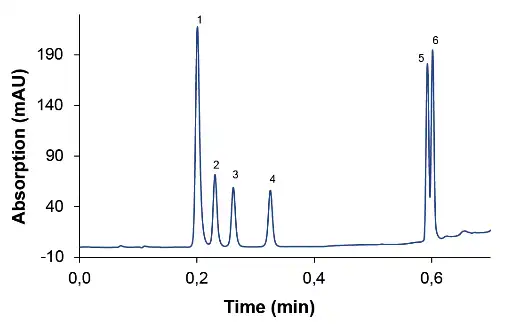
Fig. 2 Chromatogram at 1.5 ml/min, (1) caffeine, (2) PEMA, (3) ethosuximide, (4) primidone, (5) carbamazepine, (6) phenytoin
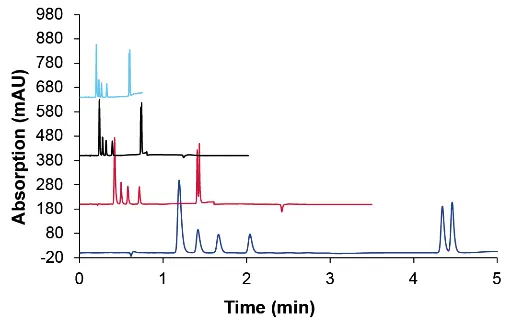
Fig. 3 Overlay of chromatograms at different flow rates, dark blue- 0.2 ml/min, red – 0.6 ml/min, black – 1.2 ml/min, light blue – 1.5 ml/min
Backpressure and run time at different flow rates
Flow rate [ml/min] | Run time [min] | Pressure [bar] |
0.2 | 7.0 | 120 |
0.6 | 3.5 | 370 |
1.2 | 2.0 | 780 |
1.5 | 1.6 | 1000 |
System Volumes
Fast and highly efficient separations require a system with low system volumes. The extra column volume (ECV) is the volume between the point of the injection and the point of detection. It relates to the band spread, and every connection, tubing, or fitting in the post-injection flow path contributes to the ECV. It should be kept small to reduce the dispersion and therefore enhance resolution. Determination of ECV was carried out with a zero dead volume union/coupling instead of a column. For detailed method parameters please refer to the materials and method section.
Several independent studies and experiments from different vendors were used for comparison of ECV of (U)HPLC systems [1], [2].
The AZURA UHPLC system was equipped with a P 8.1L pump with a 50 µl static mixer and a 10 µl filter, a zero dead volume coupling (ZDV), capillaries with 0.1 mm ID (from pump to autosampler) and 0.075 mm ID (autosampler to ZDV; ZDV to detector). The autosampler was equipped with a 10 µl sample loop. The used flow cell had a 10 mm flow path and a volume of 2 µl. The ECV of 9 µl was determined at 5σ (4.4 %) peak height.
Comparison of extra-column band (ECV) spread [1], [2], [3]
System | ECV [µl] |
System A | 13 |
System B | 14 |
System C | 14 |
System D | 12 |
System E | 10 |
Alliance® HPLC System | 29 |
Vendor A HPLC | 41 |
Vendor B1 UHPLC | 28 |
Vendor B2 UHPLC configured for Single Column | 20 |
Vendor B2 UHPLC configured for Dual Column | 23 |
Vendor C UHPLC | 21 |
Vendor D UHPLC | 17 |
ACQUITY UPLC H-Class | 9 |
ACQUITY UPLC H-Class with Column Manager | 12 |
ACQUITY UPLC I-Class (SM-FTN) | 7.5 |
ACQUITY UPLC I-Class (SM-FL) | 5.5 |
KNAUER AZURA UHPLC | 9 |
Reproducibility
To determine the reproducibility for each flow rate, 10 replicates were measured. The standard deviation of retention time for each substance and flow rate is displayed in Fig. 4.
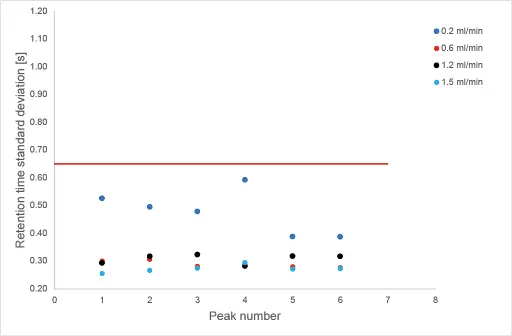
Fig. 4 Standard deviation of retention time over 10 replicates for each flow rate
The methods showed excellent reproducibility. For all applied flow rates and for all substances over the 10 replicates, the retention time shift is below 0.60 seconds.
This is a result of the adaptive pulsation compensation of the pump, which is independent of flow rate, backpressure, and eluent type through real-time eluent compressibility monitoring and variable piston stroke. Together with the ultra-precise piston movement thanks to KNAUER’s proprietary advanced piston drive technology, an outstanding flow reproducibility at any working conditions can be achieved.
Retention times at different flow rates
Peak asymmetry
With the increase of flow rate only slightly changes in peak symmetry were observed which demonstrates that the used system has constant dispersion characteristics.
Figure 5 exemplary shows a normalized, enhanced overlay of primidone at the different flow rates.
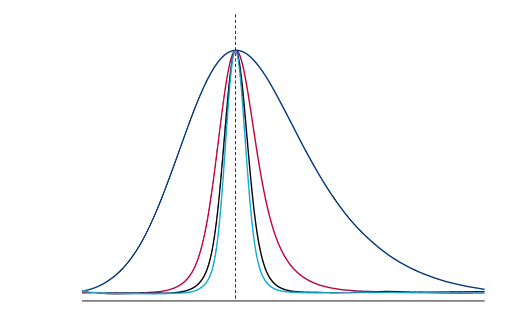
Fig. 5 Normalized overlay of primidone (peak 4), dark blue- 0.2 ml/min, red – 0.6 ml/min, black – 1.2 ml/min, light blue – 1.5 ml/min
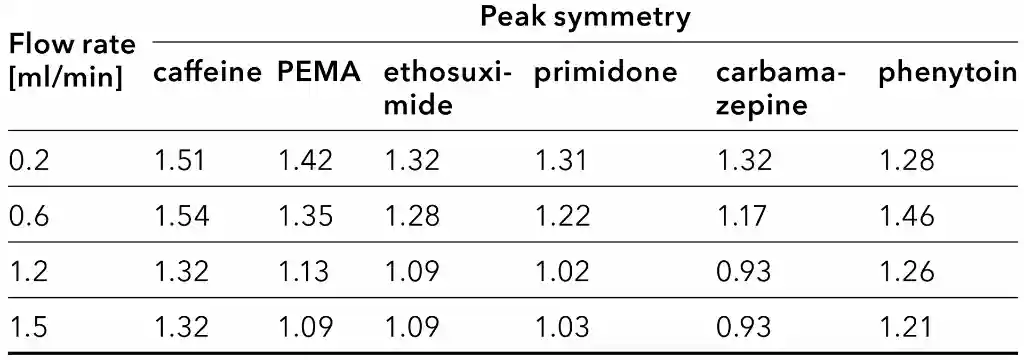
Table 4 summarizes the symmetry values for all substances and flow rates. For most of the analytes, the peak shape was improved with the increase of the flow rate.
Peak symmetry at different flow rates
Efficiency
The Van Deemter equation is an empirical formula describing the relationship between plate height, as a measure of column efficiency, and linear velocity. When plotting the flow rate versus height equivalent to a theoretical plate (HETP), a Van Deemter plot is generated which helps to determine the optimal flow rate for a chromatographic system regarding column efficiency. Figure 6 displays the empirical determined plots for the six analyzed compounds.
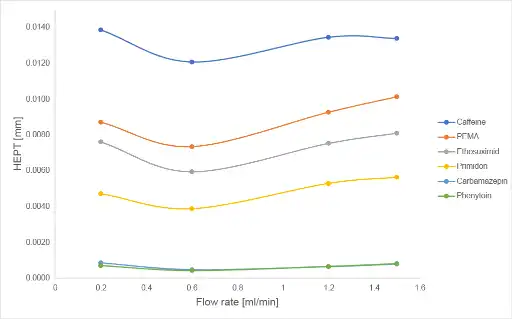
Fig. 6 Van Deemter plots
For all components the lowest HEPT values were achieved at a flow rate of 0.6 ml/min. So, this could be assumed as the optimal flow rate for this specific chromatographic system. Doubling the flow rate from 0.6 ml/min to 1.2 ml/min results in a loss of efficiency of about 10 % but also in a saving of time of 43 %.
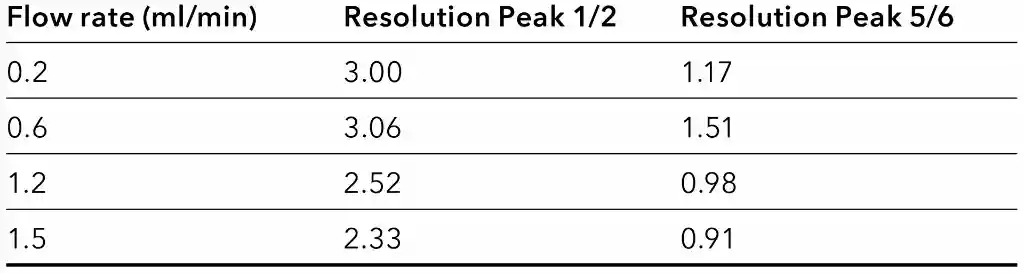
Resolution
Increasing the flow rate can also have an impact on the resolution between peaks. Most of the substances remained baseline separated regardless of the flow rate.
The most critical peaks in this application are carbamazepine and phenytoin. Table 5 exemplary shows the change in resolution for two different peak pairs. The resolution of phenytoin decreases from 1.71 at 0.2 ml/min to 0.91 at 1.5 ml/min. If this resolution is still sufficient for quantification, must be evaluated by the user.
Resolution for different peak pairs at different flow rates
Conclusion
Due to the broad variety of applicable flow rates and pressure ranges, and the low extra column volume the KNAUER AZURA UHPLC system equipped with the new binary AZURA P 8.1L pump is perfectly suitable for high throughput analysis, preserving resolution. A reduction of 77 % of total analysis time was achieved when increasing the flow rate from 0.2 ml/min to 1.5 ml/min,
Material and Methods
Tab. 6 System configuration
Instrument | Description | Article No. |
Pump | AZURA P 8.1L, HPG | |
Autosampler | AZURA AS 6.1L, 1240 bar | |
Detector | AZURA DAD 6.1L | |
Flow cell | Standard KNAUER LightGuide UV Flow Cell Cartridge, 10 mm/2 µl | |
Thermostat | AZURA CT 2.1 | |
Capillaries | MarvelXACT Peek-Lined SS 0.075 mm ID | |
350 mm | AZF05-1 | |
500 mm | AZF05 | |
AZURA® Analytical K-Connect 0.1 mm ID | ||
700 mm | ||
Software | ClarityChrom 8.7 – Workstation, autosampler control included | |
Software | ClarityChrom 8.7 - PDA extension | |
Column | Agilent Zorbax SB-C18 RRHD, 2.1x50mm, 1.8 µm | –––––– |
Tab. 7 Fixed method parameters
Parameter | Description |
Eluent A | water |
Eluent B | acetonitrile |
Temperature | 60 °C |
Detection | 204 nm |
Data rate | 100 Hz |
Time constant | 0.01 s |
Injection mode | Partial loop |
Injection volume | 2 µl |
Gradient program for different flow rates
Flow rate | 0.2 ml/min | 0.6 ml/min | 1.2 ml/min | 1.5 ml/min | ||
Gradient | Time (min) | Time (min) | Time (min) | Time (min) | A (%) | B (%) |
0.00 | 0.00 | 0.00 | 0.00 | 85 | 15 | |
2.03 | 0.61 | 0.30 | 0.24 | 78 | 22 | |
2.30 | 0.70 | 0.35 | 0.28 | 70 | 30 | |
3.41 | 1.03 | 0.50 | 0.40 | 65 | 35 | |
5.36 | 1.62 | 0.81 | 0.65 | 5 | 95 | |
5.39 | 2.10 | 1.10 | 0.88 | 85 | 15 | |
7.00 | 3.50 | 2.00 | 1.60 | 85 | 15 |
Method parameters for ECV determination
Parameter | Description |
Eluent A | acetontrile: water 70:30 (v/v) |
Flow rate | 0.3 ml/min |
Temperature | ambient |
Detection | 273 nm |
Data rate | 50 Hz |
Time constant | 0.02 s |
Injection mode | Partial loop |
Injection volume | 1 µl |
Sample | 150 µg/ml caffeine in acetontrile:water 90:10 (v/v) |
Autosampler solvent | acetonitrile:water 90:10 (v/v) |
Column | zero dead volume union |

References
[1] ACQUITY UPLC I-Class: Optimized System Dispersion for Ultimate UPLC Performance; Waters Corporation; https://www.waters.com/content/dam/waters/en/app-notes/2011/720003947/720003947-en.pdf; 24/03/2023
[2] Optimized System Dispersion for UPLC Performance in a Versatile LC Design; Waters corporation; https://www.waters.com/webassets/cms/library/docs/720003651en.pdf; 24/03/2023
[3] A 14 Parameter Study of UHPLC´s for Method Development Transfer and Troubleshooting; Ahmad et. al; DOI:10.1007/s10337-017-3337-8
Application details
|
Method |
UHPLC |
|
Mode |
RP |
|
Substances |
Caffeine, ethosuximide, primidone, carbamazepine, phenytoin, 2-ethyl-2-phenylmalonamide monohydrate |
|
CAS number |
80866-90-6, 58-08-2, 77-67-8, 125-33-7, 298-46-4, 57-41-0 |
|
Version |
Application No.: VTN0023 | Version 1 06/2023 | ©KNAUER Wissenschaftliche Geräte GmbH |


