
Science with Passion
Application No.: VCH0018
Version 1 08/2019
Mirror, mirror … – Chiral column screening for enantioseparation of α-ionone
Juliane Boettcher1, Martin Steinhaus2, Jörg Stein2, Kate Monks1; applications@knauer.net
1KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin;
2Leibniz-Institut für Lebensmittel-Systembiologie an der Technischen Universität München

Photo: Pixabay
Summary
The chiral separation of enantiomers is interesting for different fields of applications, e.g. pharmaceutical products, perfumery, and flavouring. Sometimes both enantiomers can be used for the application, sometimes only one enantiomer is of further interest. Ionones are closely related substances to the group of rose ketones which are key aroma chemicals in the successful recreation of rose scents.
Introduction
Chirality is one of the basic principles in nature. It denominates the fact, that there are items which act like image and mirror image but can´t be brought to congruence along an axis of reflection although they are similar. Human hands are the most common used example of chirality. The left hand is a non-superimposable mirror image of the right hand. In chemistry, chirality describes the steric configuration of atoms in molecules which have the same constitution. Most often the cause of chirality in molecules is the presence of an asymmetric carbon atom. The Cahn-Ingold-Prelog-convention (short: CIP-convention or (RS)-system) helps to give a distinct description of the spatial arrangement of the different bound substituents and to classify the enantiomers.
The following column screening was performed to separate the enantiomers of α-ionone. The (R)-enantiomer has a violet, raspberry and floral smell, whereas the (S)-α-ionone has a violet like and woody smell. The use of α-ionone is very multifarious. Because of its valuable violet aroma, it is used for fragrances as well as consumables like chewing gum, beverages, pastries, and sweets. [1],[2]
Three different chiral stationary phases (CSPs) were used. The Eurospher II Chiral AM/AM R is a coated phase with amylose-tris-3,5-dimethylphenyl-carbamate as chiral selector whereas the Eurospher II Chiral OM/OM-R is a cellulose tris-3,5-dimethylphenylcarbamate coated porous silica phase. The AM phase is suitable for normal phase and the AM-R can be used with reversed phase solvents (same for OM and OM-R). The third CSP used for screening, Eurospher II Chiral NR, is an immobilized brush-type phase with very broad generality and complementary selectivity to Eurospher II Chiral AM and OM. It is very stable because the Chiral-NR selector is covalently bound, so that all HPLC eluents can be used.
Results
The screening was performed under reversed phase and normal phase conditions. The samples were provided by the Leibniz-Institute for Food Systems Biology at the Technical University of Munich. A concentration of approximately 2 mg/mL of a racemic α-ionone standard was dissolved in n-hexane and in methanol. Before injection the samples were diluted in a ratio of 1:20 with methanol for reversed phase measurements and n-heptane for normal phase.
During the normal phase screening two different eluent compositions were used: n-heptane:ethanol 95:5 (v/v) and n-heptane:isopropanol 95:5 (v/v). The reversed phase measurement was also performed with two different eluents: acetonitrile and methanol. The detailed method parameters are shown in the additional materials and methods section. Under reversed phase conditions no separation was achieved for the AM-R and OM-R stationary phases. Fig. 1 shows the chromatogram of the ionone standard on the AM-R phase with methanol as eluent, where only a minimal separation is seen. Therefore, the focus was set to normal phase. Again, no enantioseparation of α-ionone was accomplished using OM and AM CSPs, but the Eurospher II Chiral NR was successful. Fig. 2 shows the measurement of α-ionone on the NR phase with n-heptane:isopropanol 95:5 (v/v) as eluent. A classification of the peaks to (R)- or (S)-α-ionone was not possible because no enantiopure standard was available. Tab. 1 summarizes the calculated values for capacity, resolution and selectivity.
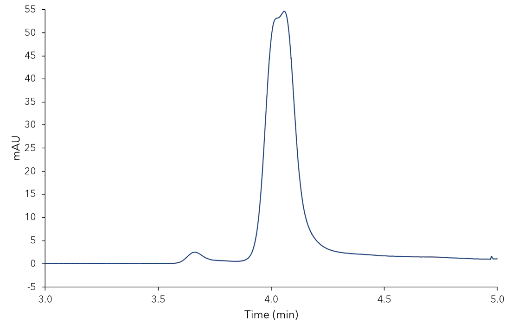
Fig. 1 Chromatogram of the α-ionone on the AM-R phase with methanol (zoom)
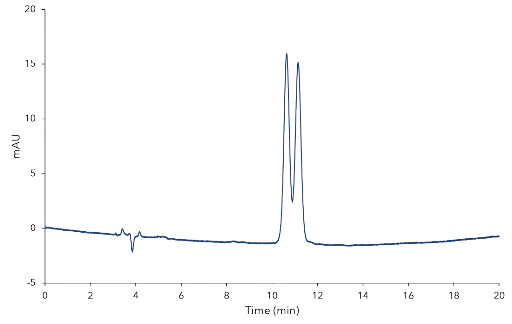
Fig. 2 Chromatogram of α-ionone on the NR phase with n-heptane:isopropanol 95:5 (v/v)
Tab. 1 Calculated values and retention times
Parameter | Value | |
unretained peak time | t0 | 3.849 min |
retention time 1 | t1 | 10.638 min |
retention time 2 | t2 | 11.147 min |
capacity factor 1 | k1 | 1.7638 |
capacity factor 2 | k2 | 1.8961 |
selectivity | α | 1.0750 |
resolution | R | 1.0680 |
Materials and Methods
Two configurations of AZURA analytical systems were used. The normal phase screening was performed with an isocratic AZURA P 6.1L for normal phase applications in combination with the GPC degasser. In reversed phase mode an AZURA P 6.1L HPG pump was used. All other devices were the same for both systems: AZURA CT 2.1 thermostat, autosampler AZURA AS 6.1L, AZURA DAD 2.1L. All measurements ran isocratic at flow rate of 0.7 mL/min. The detection wavelength was set to 220 nm and 3D data were acquired. An additional assistant AZURA ASM 2.1L was used for column switching in the reversed phase screening system. All data were recorded using the ClarityChrom 8.1 software.
References
[1] http://www.thegoodscentscompany.com/data/rw1011952.html, 07.08.2019
[2] https://www.internetchemie.info/chemie-lexikon/stoffe/a/alpha-isomethyl%20ionone.php, 07.08.2019
AZURA HPLC Plus system configuration for normal phase
AZURA HPLC Plus system configuration for reversed phase
Conclusion
The screening process contained the verification of three stationary phases: Chiral-NR, Chiral-AM/AM-R and Chiral-OM/OM-R. The aim of the screening was to find an appropriate CSP for the separation. A successful chiral separation of (R)- and (S)-α-ionone was realised using the Eurospher II Chiral NR phase under normal phase conditions. A further method optimization could possibly improve the resolution. The application of n-heptane without the addition of alcohols would be reasonable but will lead to longer retention times. Another alternative would be the screening of further, more special, chiral stationary phases.
Additional Materials and Methods
Tab. A1 Method parameters
Column temperature | 25 °C |
Injection volume | 1 µL |
Injection mode | Partial Loop |
Detection | UV 220 nm |
Data rate | 10 Hz |
Tab. A2 Pump parameters (normal phase)
Eluent 1 | n-heptane:isopropanol 95:5 (v/v) |
Eluent 2 | n-heptane:ethanol 95:5 (v/v) |
Gradient | isocratic |
Flow rate | 0.7 mL/min |
Tab. A3 Pump parameters (reversed phase)
Eluent 1 | methanol |
Eluent 2 | acetonitrile |
Gradient | isocratic |
Flow rate | 0.7 mL/min |
Tab. A4 System configuration
Instrument | Description | Article no. |
Pump | AZURA P6.1L, isocratic NP | |
Pump | AZURA P6.1L HPG, RP | |
Degasser | 2 channel GPC degasser | |
Autosampler | AZURA AS 6.1L | |
Detector | AZURA DAD 2.1L | |
Thermostat | AZURA CT 2.1 | |
Assistant (optional) | ASM 2.1L, Left: 6Mpos, 1/16", sst, 300 bar middle: 6Mpos, 1/16", sst, 300 bar right: empty module | |
Column 1 | Eurospher II Chiral AM, 250 x 4 mm ID with precolumn | |
Column 2 | Eurospher II Chiral AM-R, 250 x 4 mm ID with precolumn | |
Column 3 | Eurospher II Chiral OM, 250 x 4 mm ID with precolumn | |
Column 4 | Eurospher II Chiral OM-R, 250 x 4 mm ID with precolumn | |
Column 5 | Eurospher II Chiral NR, 250 x 4 mm ID with precolumn | |
Software | ClarityChrom 8.1 – workstation, autosampler control included | |
Software | ClarityChrom 8.1 – PDA extension |
Related KNAUER Applications
VCR0058J – Chiral separation of p-Menthadienol ((1R/S,4R)-1-methyl-4-(prop-1-en-2-yl)cyclohex-2-enol)
VCR0029J – Chiral separation of Flavanone (2-Phenyl-1,4-Benzopyrone)
Application details
Method | HPLC |
Mode | Chiral |
Substances | CBC, CBCA, CBDV, CBDVA, CBD, CBDA, CBG, CBGA, CBN, CBNA, CBL, CBLA, Δ9-THC, Δ9-THCA, Δ8-THC, THCV, THCVA |
CAS number | 127-41-3 (rac), 24190-29-2 (R), 14398-36-8 (S) |
Version | Application No.: VCH0018 | Version 1 08/2019 | ©KNAUER Wissenschaftliche Geräte GmbH |


