
Science with Passion
App-Nr.: VBS0079
Version 1 03/2021
Charakterisierung von Fructanen in Fermentationsprodukten mit GPC/SEC
Johannes Menke, Yannick Krauke, Kate Monks; applications@knauer.net
KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin
Stanislav Parsin, Technische Universität Hamburg
Zusammenfassung
Jüngste Bemühungen zur Verbesserung der Effizienz von Biorefinereien zielen darauf ab, die ansonsten verworfenen Nebenprodukte zu nutzen. In dieser Anwendung wurde eine Probe biotechnologischen Ursprungs von der Becherpflanze (Silphium perfoliatum) hinsichtlich der Verteilung von Fructanpolymeren und anderen Zuckergehalten charakterisiert. Dies wurde unter Verwendung der Größenausschlusschromatographie (SEC) in Kombination mit der Brechungsindexdetektion (RID) erreicht. Anschließend wurde die Methode auf eine semi-präparative Dimension skaliert, um Oligomere von Monomeren zu trennen.
Einführung
In vielen Fällen ist einer der letzten Schritte eines biotechnologischen Prozesses die Reinigung von Zielverbindungen mit einer Form des LC-Prozesses. In der Produktionsphase ist zudem eine Prozesskontrolle erforderlich. Daher wird in vielen Fällen LC in einer oder anderen Form in biotechnologischen Prozessen angewendet. Proben oder Produkte, die aus einem biotechnologischen Prozess auf Basis von Becherpflanzen (Silphium perfoliatum) [1] stammen, werden voraussichtlich Fruktane unterschiedlicher Länge sowie andere Zucker und Monomere [2] enthalten. Eine qualitative Charakterisierung wurde unter Verwendung der Größenausschlusschromatographie (SEC) in Kombination mit der Brechungsindex (RI)-Detektion durchgeführt. Die Probe wurde hinsichtlich der Molekulargewichtsverteilung bestimmt. Eine universelle Kalibrierung mit Polyethylenglykol (PEG)-Standards wurde verwendet, um festzustellen, ob die Probe Moleküle im Molekulargewichtsbereich der erwarteten Fruktane enthielt. Die Methode wurde auf eine semi-präparative Dimension hochskaliert, um die Fruktan-Oligomerfraktion von den niedrigmolekularen Zuckern zu isolieren. Darüber hinaus wurden die präparativen Experimente genutzt, um die Machbarkeit der Anwendung eines kontinuierlichen Gegenstrom-LC-Prozesses, respektive der simulierten bewegten Betchromatographie (SMB), zu bewerten. Für diese Prozesse ist eine binäre Trennung erforderlich.
Ergebnisse
Die universelle Kalibrierung wurde erfolgreich etabliert. Die Kalibrierkurve, eine polynomiale Funktion fünfter Ordnung, wurde an die zehn Messungen mit einem Bestimmtheitsmaß R² von 0,99978 angepasst, unter Verwendung der Mark-Houwink-Parameter K=10,4 und α=0,57 (Abb. 1).
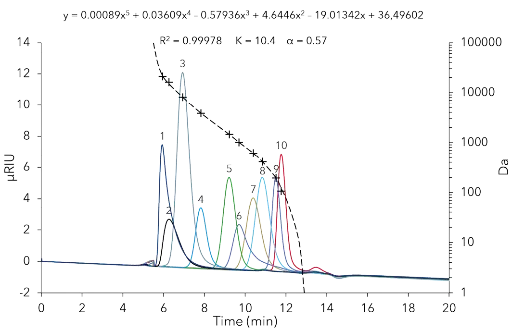
Abb. 1 Kalibrierungsüberlagerung einzelner Standards. 50 µl Injektionsvolumen, Flussrate 1 ml/min, 25 °C. 1 - MP21160, 2 - MP16100, 3 - MP8160, 4 - MP3860, 5 - MP1450, 6 - MP1010, 7 - MP610, 8 - MP410, 9 - MP194, 10 - MP106.
Daher konnten in den folgenden Experimenten Substanzen mit einem Molekulargewicht zwischen 106 Da und 21 160 Da bestimmt werden. Die stationäre Phase AppliChrom ABOA-SuperOH-P mit einer Partikelgröße von 7 µm und einer Porosität von 250 Å wurde gewählt, da sie optimal für wässrige Trennungen ist. Diese Größenausschlussphase kann für Analytika mit einem Molekulargewicht von bis zu 70 000 Da verwendet werden. Das Ausgangsmaterial ist ein hydrophiler Polymer, der in einem pH-Bereich von 2,5 – 12 stabil ist und Drücke von bis zu 80 bar sowie Temperaturen von 10 bis 85 °C standhalten kann. Die Probe wurde mit 50 µl einer 1:20 Verdünnung analysiert. Bei einer Flussrate von 1 ml/min und einer Temperatur von 25 °C wurde eine ausreichende Trennung der Oligomere (Peak 1) und niedermolekularer Zucker (Mono-, Di-, Trimere) (Peak 2) erreicht (Abb. 2).
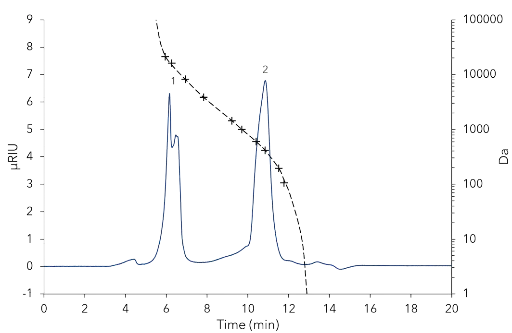
Abb. 2 Probe im analytischen System mit 8 mm ID-Säule. 50 µl Injektionsvolumen, 1:20 Verdünnung, Flussrate 1 ml/min, Temperatur 25 °C. 1 - Fructan-große Moleküle (Oligomere ~112 Einheiten), 2 - Niedermolekulare Zucker.
Der Oligomerpeak wurde mit einem durchschnittlichen Molekulargewicht von 20 247 Da bestimmt. Aus diesem Ergebnis kann eine ungefähre Kettenlänge von 112 Einheiten abgeleitet werden, basierend auf dem Molekulargewicht von monomerem Fructose (MW 180,16 Da). Die Methode wurde auf eine semi-präparative Skala mit der KNAUER ScaleUp Converter-Software hochskaliert (A1696 Bei konstanter Säulenlänge und Partikelgröße war eine lineare Skalierung (wenn auch nicht wirklich linear aufgrund der Vor-Säule mit einer Länge von 50 mm) durch Erhöhung des inneren Säulendurchmessers (8 mm ID auf 20 mm ID) und der Flussrate (1 ml/min auf 7 ml/min) möglich. Für die präparativen Experimente wurden 200 µl der unverdünnten Probe injiziert. Die Ergebnisse zeigten eine ausreichende Auflösung zwischen oligomeren und niedermolekularen Zuckern (Abb. 3). Zur Fraktionierung der Probe wurde das Chromatogramm in 7,5 ml Fraktionen mit einem Fraktionierer geschnitten. Die interessierenden Fraktionen wurden mit dem analytischen System analysiert. Die Fraktionen F6 und F7, von denen erwartet wurde, dass sie die Zielsubstanzen enthalten, erwiesen sich hauptsächlich als Oligomere. F6 wies eine höhere Reinheit auf als F7 (Abb. 4).
Ergebnisse
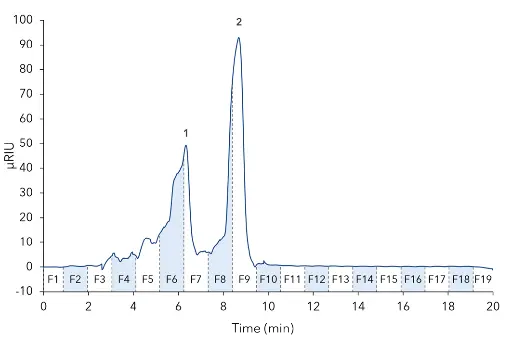
Abb. 3 Probe im semi-präparativen Maßstab mit einer 20 mm ID-Säule. 200 µl Injektionsvolumen, unverdünnt, Flussrate 7 ml/min, Temperatur 25 °C. 1 - Fructan-große Moleküle (Oligomere ~112 Einheiten), 2 - Niedermolekulare Zucker.
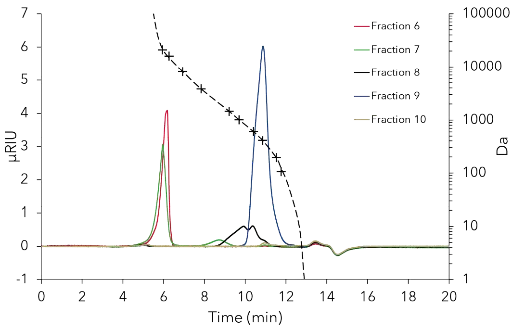
Abb. 4 Fraktionsanalyse im analytischen System. 100 µl Injektionsvolumen, unverdünnt, Flussrate 1 ml/min, Temperatur 25 °C.
Probenvorbereitungen
Für die universelle Kalibrierung wurde ein PEG-Narrow-Standardkit von Agilent mit zehn Standards verwendet. Eine Serie von zehn Standards wurde mit einer Reihe von Molekulargewichten Mp von 106, 194, 410, 610, 1 010, 1 450, 3 860, 8 160, 16 100 und 21 160 Da vorbereitet. Zu diesem Zweck wurde aus jedem Standard eine 0,1 %ige Lösung (w/v) unter Verwendung von destilliertem Wasser hergestellt. Die gefrorene Prozessprobe, die bei -20 °C gelagert wurde, wurde bei Raumtemperatur aufgetaut. Anschließend wurden 15 ml der Probe bei 10 000 g für 30 Minuten bei Raumtemperatur zentrifugiert. Der Überstand wurde dekantiert und das Pellet verworfen. Der Überstand wurde durch eine Membran aus regenerierter Cellulose (RC) mit einer Porengröße von 0,45 µm filtriert. Für die präparative Verwendung war die Probe injektionsbereit, für die analytische Bestimmung wurden mehrere Verdünnungen (1:10, 1:20, 1:50, 1:100) unter Verwendung von destilliertem Wasser hergestellt.
Fazit
SEC wurde effektiv an einer Fructan-haltigen Probe eingesetzt, um Oligo- von niedermolekularen Zuckern zu trennen. Mit der beschriebenen Analysemethode kann eine qualitative Analyse bezüglich der Molekülgröße bereitgestellt werden. Die RI-Detektion ist robust und empfindlich genug, um die Zuckersignale zu erfassen. Die universelle Kalibrierung kann leicht an andere Proben mit unterschiedlichen Analyten/Lösungsmitteln angepasst werden, indem die Mark-Houwink-Parameter K und α gemäß der Literatur (z. B. Polymerhandbücher) geändert oder Parameter verwendet werden, die von der ClarityChrom-Software vorgeschlagen werden. Eine weitere Möglichkeit zur Optimierung der Annäherung an die Molekulargewichte wäre die Auswahl von Standardsubstanzen mit einem hydrodynamischen Radius, der näher an den Analyten liegt. Die Skalierung des Prozesses wurde leicht mit der KNAUER ScaleUp Converter-Software [3] erreicht. Die Fraktionierung zeigte, dass Oligomere (F6 und F7) erfolgreich von den niedermolekularen Zuckern/Komponenten (F8 und F9) isoliert wurden. Im präparativen Maßstab war die Auflösung zwischen der oligomeren und der niedermolekularen Fraktion ausreichend, um einen SMB-Entwicklungsprozess zu ermöglichen, da die Probe in ein binäres Gemisch getrennt wurde. Obwohl SEC einige Nachteile für die präparative Reinigung hat, wie kleinere Ladevolumina und niedrigere Flussraten im Vergleich zu silikabasierten stationären Phasen, ist die strikte größenbasierte Trennung ihr Hauptvorteil für die gegebene Trennaufgabe.
Referenzen
Mayr, J., von Gehren, P., Gansberger, M., Hösch, J., Liebhard, P., Montgomery, L., Pachinger, B., Gautam, I. Das Potenzial der Energiepflanze Silphium perfoliatum L.. 23. Europäische Biomasse-Konferenz und Ausstellung, Wien, Österreich (2015).
Pollock, C.J., Chatterton, N.J.. Fructane. In: Stumpf, P.K. und Conn, E.E. Die Biochemie der Pflanzen: Ein umfassendes Handbuch. 14/109-140, Academic Press, Inc. (2012).
[3] KNAUER ScaleUp Konverter.
https://www.knauer.net/software-hplc-method-converter.
Materialien und Methoden
Tab. 1 Methodenparameter – Analytische HPLC
Kolonnentemperatur | 25 °C |
Injektionsvolumen | 50 µl |
Injektionsmodus | Teilweise Schleife |
Erkennung | RI |
Detektortemperatur | 35 °C |
Datenrate | 10 Hz |
Eluent | Wasser |
Durchflussrate | 1 ml/min |
Pumpenprogramm | Isokratisk |
Tab. 2 Systemkonfiguration – Analytische HPLC
Instrument | Beschreibung | Artikel-Nr. |
Pumpe | AZURA P6.1L, isokrat | |
Autosampler | AZURA AS 6.1L | |
Detektor | AZURA RID 2.1L | |
Thermostat | AZURA CT 2.1 | |
Spalte | AppliChrom ABOA SuperOH-P-250 300x8 mm, ID 7 µ, 100 – 70 000 Da | |
Spalte | AppliChrom ABOA SuperOH-P-250 50x8 mm, ID 7 µ, 100 – 70 000 Da pre-column | |
Rohrleitung | K-Connect 0.18 mm ID | |
Software | ClarityChrom 8.2.3 Station | |
Software | ClarityChrom 8.2.3 SEC/GPC Erweiterung | |
Software | KNAUER ScaleUp Konverter |

Tab. 3 Methodenparameter – Preparative HPLC
Kolonnentemperatur | 25 °C |
Injektionsvolumen | 200 µl |
Injektionsmodus | Full loop |
Erkennung | RI |
Detektortemperatur | 35 °C |
Datenrate | 2 Hz |
Eluent | Wasser |
Durchflussrate | 7 ml/min |
Pumpenprogramm | Isokratisk |
Fractionation | 7.5 ml slices, time-based |
Tab. 4 Systemkonfiguration – Preparative HPLC
Instrument | Beschreibung | Artikel-Nr. |
Pumpe | AZURA P6.1L, isokratische 50 ml Pumpenkopf sst | |
Ventilantrieb | AZURA Ventilvereiniger VU 4.1 | |
Ventil | 2-Wegeventil mit 6 Anschlüssen | |
Detektor | AZURA RID 2.1L Hochdurchfluss | |
Thermostat | AZURA CT 2.1 | |
Spalte | AppliChrom ABOA SuperOH-P-250 | Auf Anfrage |
Fraktionensammler | Foxy R1 | |
Rohrleitung | 0.5 mm ID PEEK | |
Software | ClarityChrom 8.2.3 Station | |
Software | ClarityChrom 8.2.3 | |
Software | KNAUER ScaleUp Konverter |

Ähnliche KNAUER-Anwendungen
VFD0172 – A D E K - Einfache Trennung von fettlöslichen Vitaminen mit GPC/SEC
VBS0066 – Schnelle und empfindliche Größenausschlusschromatographie von IgG-Antikörpern
Anwendungsdetails
|
Methode |
HPLC |
|
Modus |
GPC/SEC |
|
Substanzen |
Fructan-Polymere |
|
CAS-Nummer |
n/a |
|
Version |
App-Nr.: VBS0079 | Version 1 |


