Science with Passion
Application No.: VTN0043 Version 1 07/2025
Boosting GPC/SEC data reproducibility and accuracy with a flow marker
J. Wesolowski, J. Kramer, J. Menke;
wesolowski@knauer.net
KNAUER Wissenschaftliche Geräte GmbH, Hegauer Weg 38, 14163 Berlin
Illustration: KNAUER
Summary
This technical note demonstrates how the use of a flow marker (FM) can significantly improve the data quality of GPC/SEC (Gel Permeation Chromatography/Size Exclusion Chromatography) analyses. Therefore, three different aqueous
GPC/SEC experiments were performed to simulate how flow rate fluctuations influence the results and how a FM can help reduce these effects. The results show that without the use of a FM, fluctuations in the flow rate can lead to distorted and inaccurate data. However, these errors could be significantly reduced with a FM and the corresponding correction. Overall, the implementation of a FM is crucial to improve the reproducibility and accuracy of GPC/SEC measurements.
Introduction
GPC/SEC (Gel Permeation Chromatography/Size Exclusion Chromatography) is a liquid chromatographic technique used to determine important properties of macromolecules, such as molecular weight. Calibration is an essential part of GPC/SEC, as it enables the accurate determination of molecular weight. This involves measuring the retention time also called elution volume of various calibration standards with known molecular weights to create a calibration curve that describes the relationship between molecular weight and elution volume (Fig. 1).
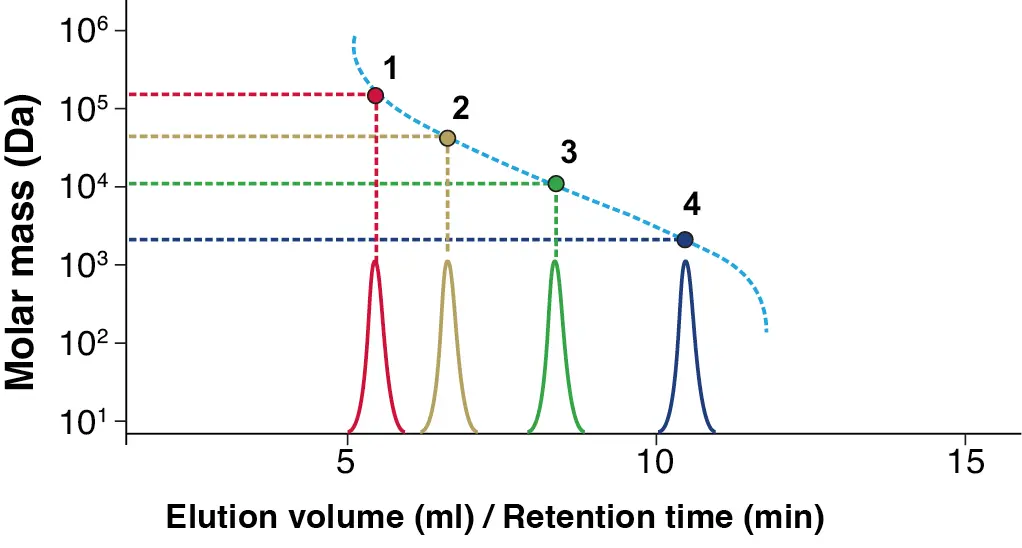
Fig. 1 Exemplary GPC/SEC calibration, where the blue peak refers to the analyte with the smallest and red to the one with the biggest molar mass in the separation range of these calibration.
To ensure the accuracy of molar mass determinations, it is crucial to maintain a constant flow rate during the measurement. Minor fluctuations in elution volume have the potential to result in significant deviations in molar mass values. In this context, a flow marker (FM) can help to increase the reproducibility and accuracy of GPC/SEC results. By providing a reference peak within each chromatogram (Fig. 2), the FM allows the detection of fluctuations in elution conditions. Consequently, correction factors can be applied to account for variations in flow rate, ensuring that retention times and elution volumes are consistent across measurements. This approach facilitates precise calibration and reliable molar mass determination, ultimately enhancing the robustness of the analytical method. In principle, any substance can be used as a FM. However, for optimal application, it is recommended to select substances with a narrow distribution and with a hydrodynamic radius close to the exclusion limit of the column to avoid overlaps with other peaks in the chromatogram. Ideally, the FM should also be soluble in the same solvent as the standards and samples. Examples include ethylene glycol for aqueous applications and ethylbenzene or butylated hydroxytoluene (BHT) for organic solutions. The substance is added at an appropriate concentration during sample and standard preparation, resulting in a distinct peak visible in the chromatogram (Fig. 2).
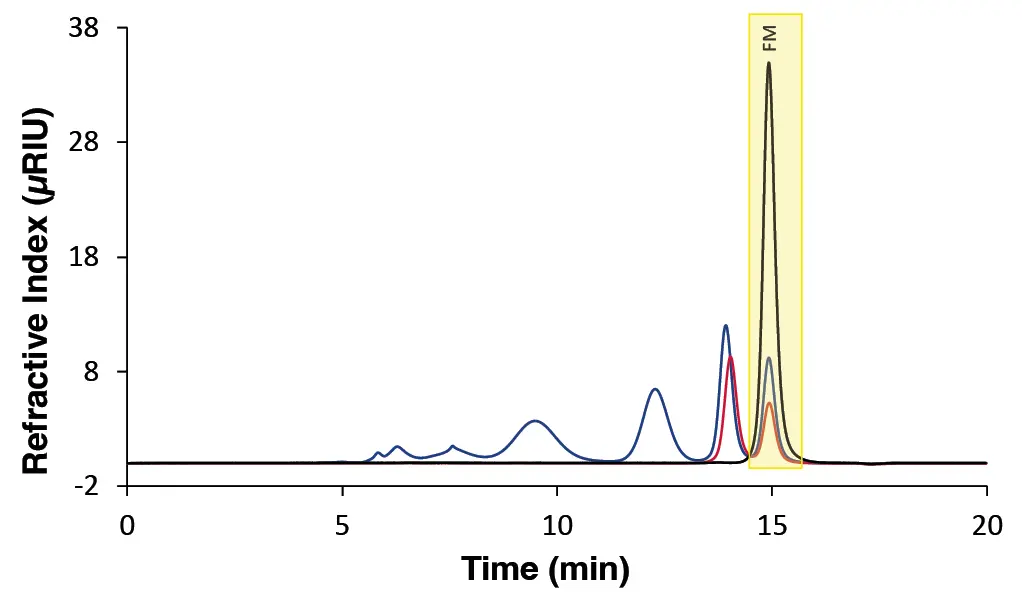
Fig. 2 Chromatogram showing different measurements with FM overlaid. Black: Ethylene glycol (FM). Red: Maltotetraose with FM. Blue: Calibration standard with FM. Yellow marking: FM peak.
Sample Preparation
For calibration with pullulan, an ReadyCal-Kit in the molar mass range 180 to
1 450 000 Da from Agilent was used. The standards were dissolved in water and left standing for one hour to assure complete dissolution. Glucose, maltose, maltotriose and maltotetraose were dissolved in water overnight at room temperature without stirring to a concentration of 2 mg/ml and then filtered through a 0.45 μm nylon filter. For all analyses, ethylene glycol was used as FM at a concentration of 1.5 mg/ml.
Results
Software
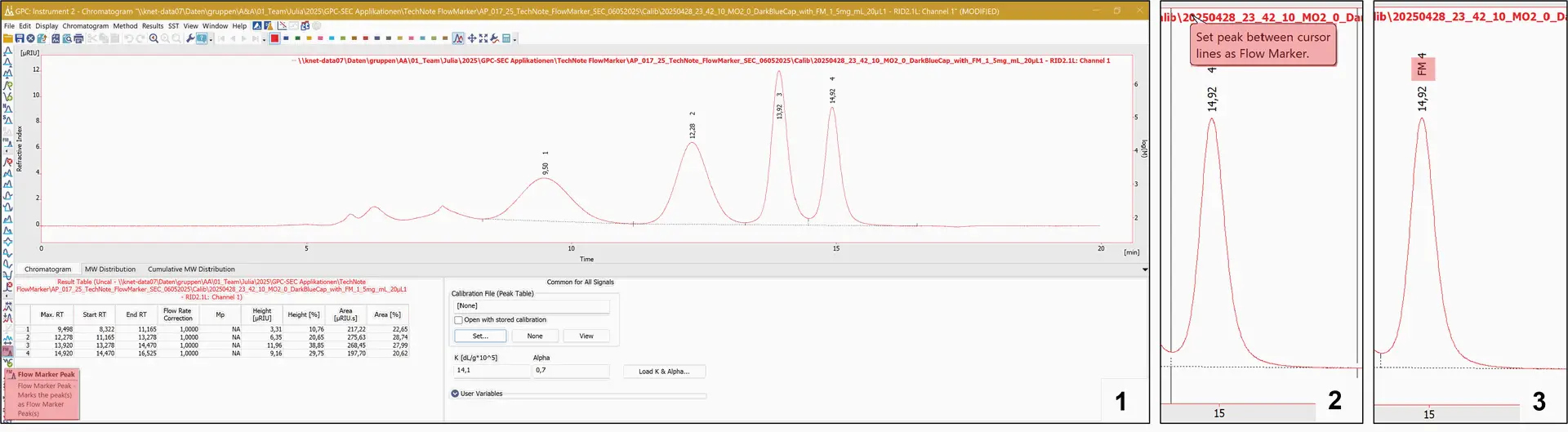
The ClarityChrom® software, with the GPC extension, enables the use of special features for peak integration and marking, including the FM, for the detection and correction of flow rate changes (Fig. 3).
Fig. 3 FM marking in the chromatogram window of the ClarityChrom® with GPC extension.
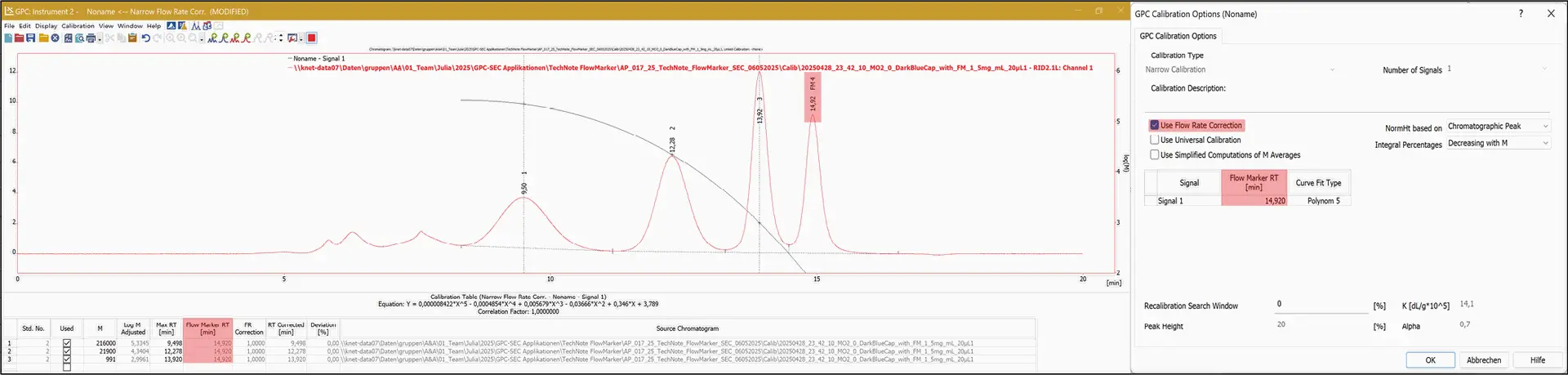
In the Calibration Window under Calibration Options, the flow rate correction can be activated, and the retention time of the FM peak can be entered (Fig. 4).
Fig. 4 Using the flow rate correction function in the calibration window of the ClarityChrom® with GPC extension.
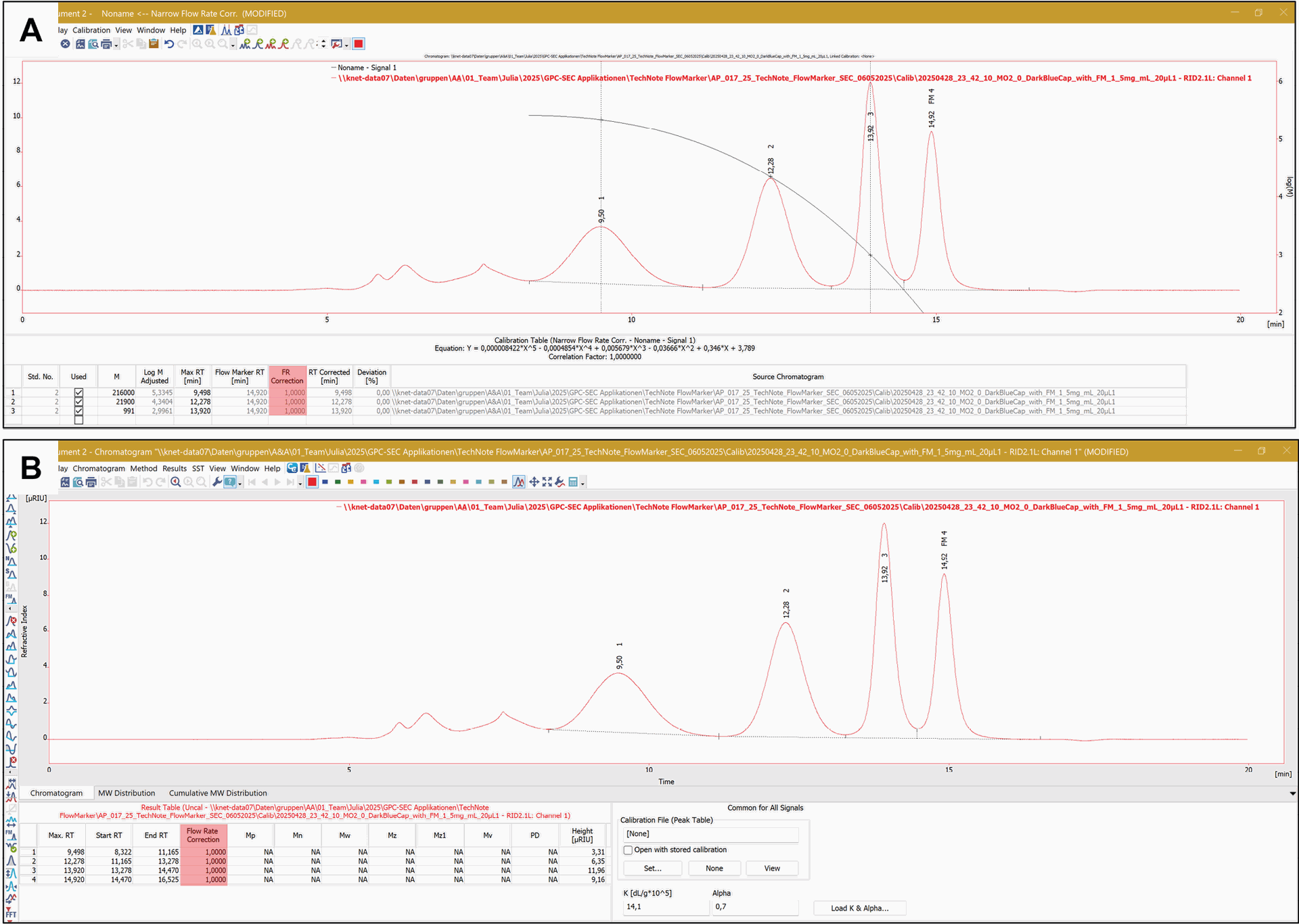
The activation is indicated in the title bar of the calibration table and the chromatogram results table by the inscription “FR Correction” and “Flow Rate Correction” (Fig. 5). Once the measurements are repeatable and there are no fluctuations in the retention time or elution volume of the FM, the correction factor is exactly 1, and no corrections are applied (Fig. 5). If the FM appears earlier in the chromatogram of a measurement it indicates a higher flow rate than set. In the calibration table (Fig. 5A) this corresponds to a correction factor > 1, while in the chromatogram results table (Fig. 5B) a value < 1 is displayed. Conversely, if the FM elutes later, it indicates a lower flow rate, resulting in a correction factor < 1 in calibration table (Fig. 5A) but showing a value > 1 in the chromatogram results table (Fig. 5B).
Fig. 5 A: “FM correction” column in the calibration table. B: “Flow Rate Correction” column in the chromatogram results table.
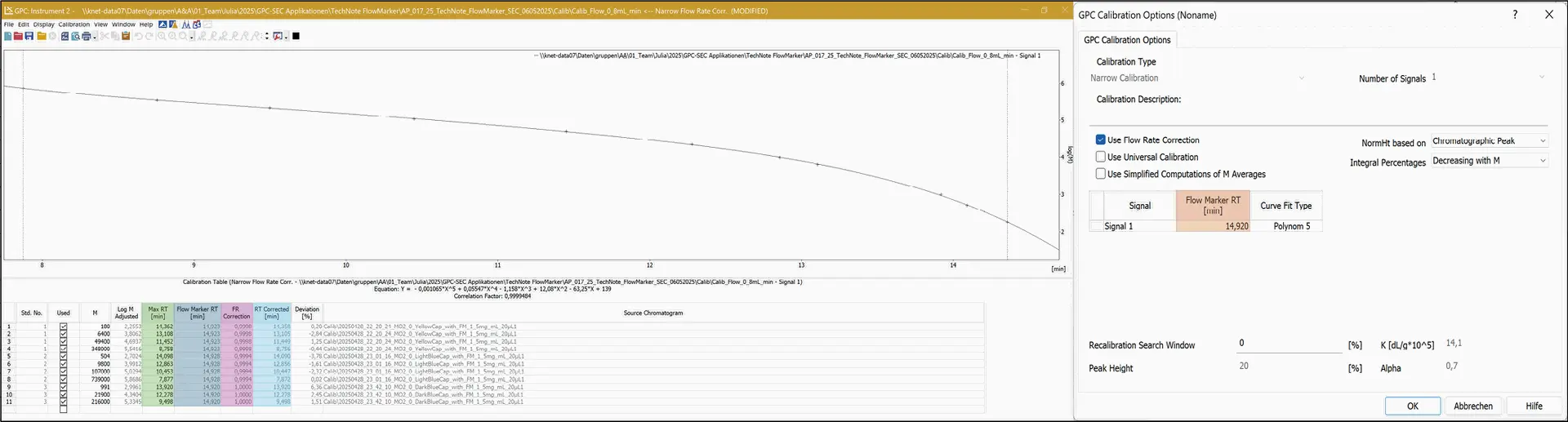
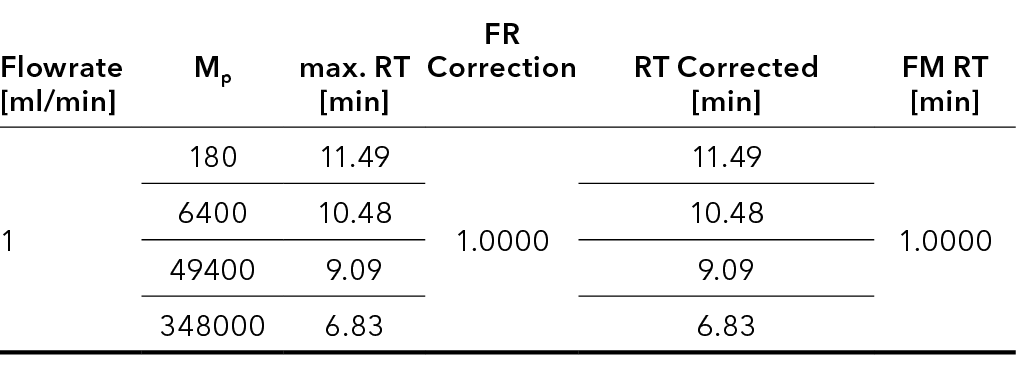
In the calibration window, the retention times of the standard measurements are corrected (Eq. 2) using the “FR correction” factor (Eq. 1).

The calibration curve is then generated using these corrected values, referred to as “RT Corrected” in the calibration table (Fig. 6).
Fig. 6 Flow rate correction within a calibration.
If sample measurements in which a FM was used are evaluated using an established calibration curve, the curve is adjusted by the “Flow Rate Correction” factor (Eq. 3). In this way, an individual calibration curve is generated for each chromatogram using the newly calculated retention times of the calibration standards (Eq. 4).
This ensures that the correct molar masses are calculated accurately despite flow rate fluctuations (Fig. 7A). Conversely, if no FM is used, or if the FM peak is not marked as such, the calibration curve will not be adjusted (Fig. 7B). This means that any fluctuations in the flow rate will not be considered, resulting in incorrect molar mass calculations (Fig. 7B).
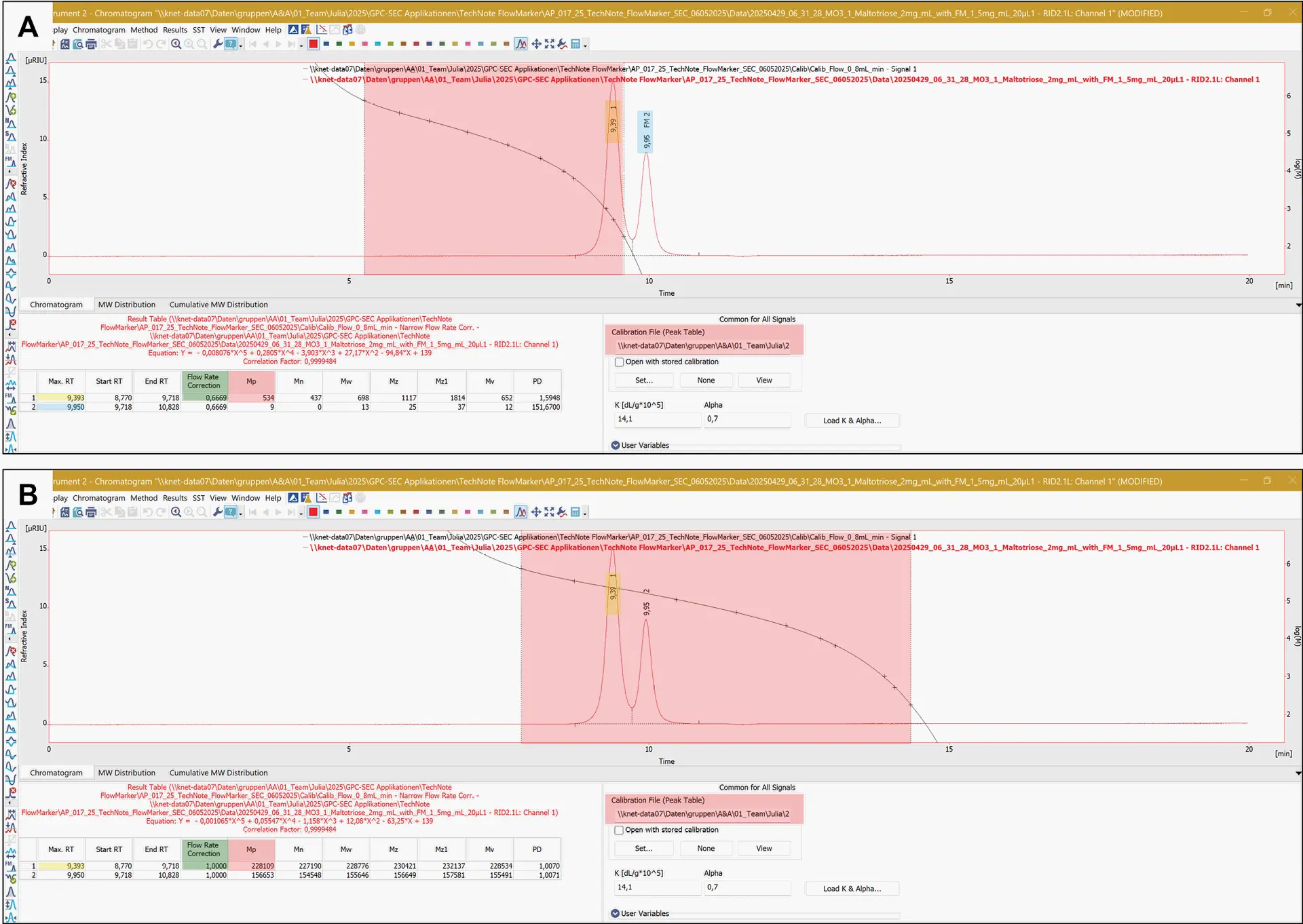
Fig. 7 Chromatogram of maltotriose (Mp, real 504 Da) with FM. A: Chromatogram of maltotriose with stored calibration and flow rate correction using a FM (Mp, calculated 534 Da). B: Chromatogram of maltotriose with stored calibration with unmarked FM following without flow rate correction (Mp, calculated 228 109 Da).
Experiments
Three experiments were carried out to test if the use of a FM can help to compensate flow rate fluctuations and thereby improve measurement accuracy and reproducibility in GPC/SEC analyses. The experiments were carried out with and without a flow rate correction using ethylene glycol as FM.
Experiment A
In the first experiment, flow rate fluctuations were simulated to determine their effects on the creation of a calibration curve. Three different pullulan standard mixes (dark blue, light blue and yellow cap) were measured to create a calibration curve. For two of the mixes (dark blue and light blue cap), a flow rate change was simulated by increasing the flow rate from 1 ml/min to 1.2 ml/min. Fig. 8 shows the two calibration datasets A and B, with and without flow rate correction. The curve fit type was selected according to the highest R² value to ensure the best possible model fit to the calibration data.
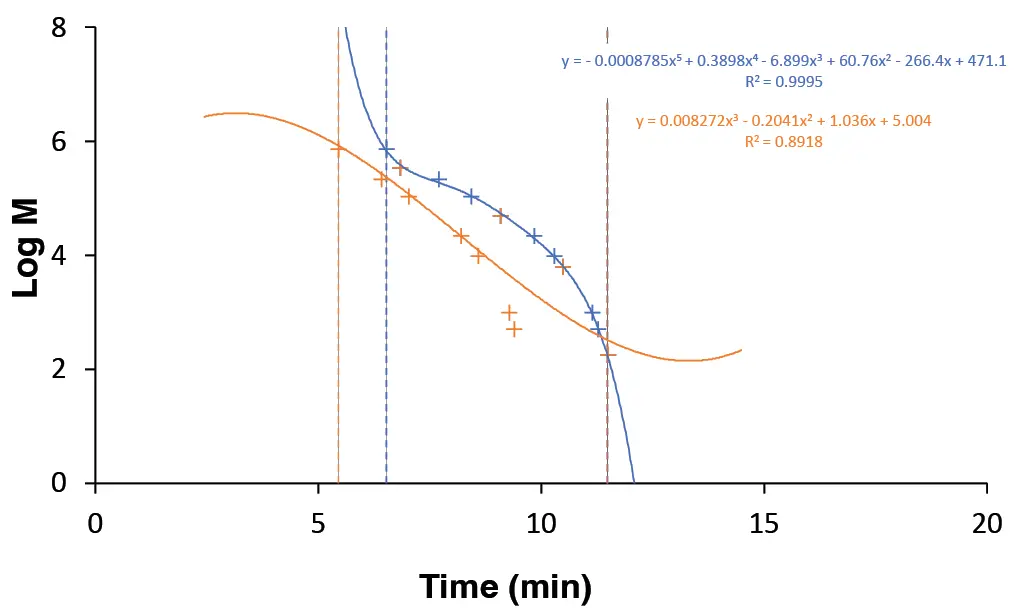
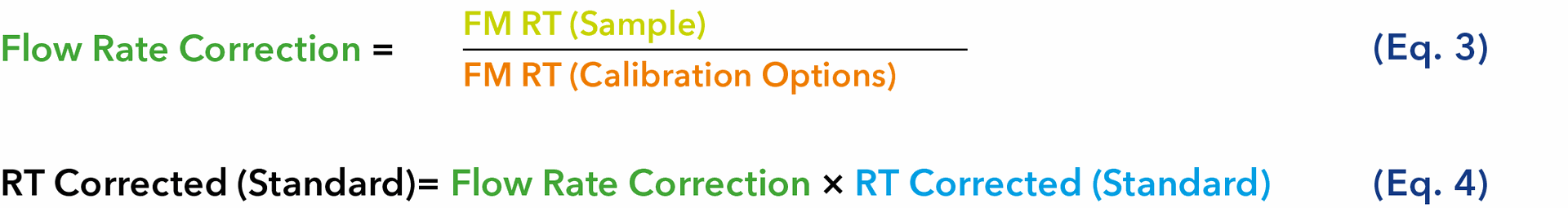
Fig. 8 Blue: Calibration A, applying FR correction; fitted using a 5th-order polynomial regression (R² = 0.9995). Orange: Calibration B, without FR correction; fitted using a 3th-order polynomial regression (R² = 0.8918).
The measurements, for which a flow rate correction was applied, show a high R² value of 0.9995. This indicates that the calibration model fits the measurement data to a high degree of accuracy and highlights the effectiveness of the flow rate correction. In contrast, the calibration data, for which no flow rate correction was performed, show a much lower R² value of 0.8918. This indicates a less precise model fit, which can be explained by the unapplied flow rate correction.
To evaluate the accuracy of booth calibrations in detail, Fig. 9 shows the percentage deviation of the individual calibration points from the respective calibration curve. Calibration A with flow rate correction (represented by the blue bars) demonstrates a high level of accuracy, with average deviations of around ±
7 %. In contrast, calibration B without flow rate correction (represented by the orange bars) shows significant deviations, particularly at calibration points 3, 7 and 10, where the deviations are over 100 %.
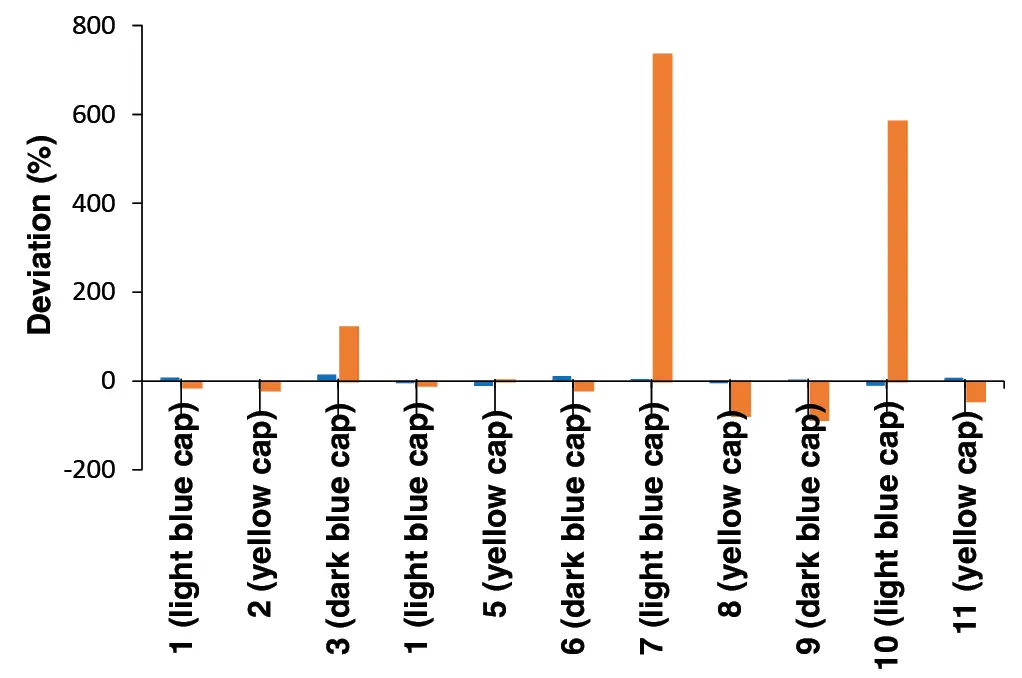
Fig. 9 Percentage deviation of calibration points (1 - 11) from the respective calibration curve. Blue: Deviation from calibration A (with flow rate correction). Orange: Deviation from calibration B (without flow rate correction).
These results demonstrate that the absence of flow rate correction leads to significant deviations, reflected in a markedly lower coefficient of determination (R²), and highlights the importance of flow rate correction for accurate molecular weight determination.
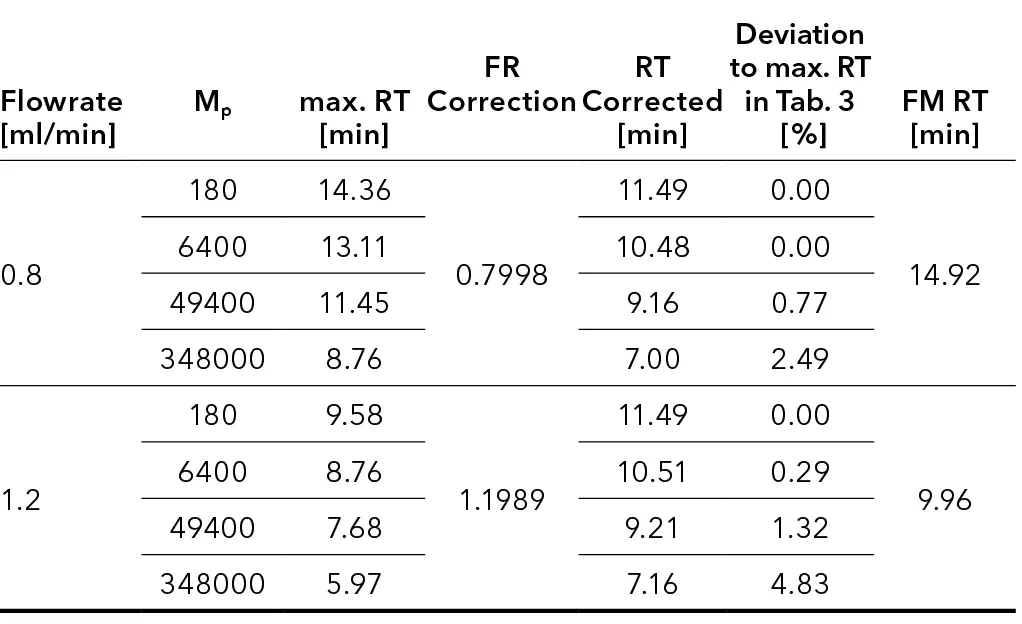
Experiment B
The second experiment evaluated the accuracy of the correction calculations by measuring a standard mixture (yellow cap) with a FM at three different flow rates. The set flow rate was 1 ml/min, while 0.8 ml/min and 1.2 ml/min were applied to simulate pump problems (under- and over-pumping). The retention times measured at these deviating flow rates were subsequently corrected using the FM. The results show that the corrected retention times (Tab. 2) closely match those at the original flow rate of 1 ml/min (Tab. 1). The deviations of the corrected retention times from those measured at a flow rate of 1 ml/min are less than 5 %, confirming the accuracy of the correction calculations (Tab. 2).
Tab. 1 Retention times of one standard mix (yellow cap) with FM measured at
1 ml/min
Tab. 2 Retention times of one standard mix (yellow cap) with FM measured at 1 ml/min
Experiment C
In the last experiment the effect on the molar mass determination was investigated if the flow rate during the sample measurement differs from the one used in the calibration. Therefore, four sugars with known molar masses were measured at a flow rate of 1.2 ml/min and evaluated using Calibration A from Experiment A, which was established at a flow rate of 1 ml/min. The results showed that the flowrate difference of + 0.2 ml/min resulted in an overestimation of the molar mass (represented by the green bars and green line) (Fig. 10). This effect could be corrected by using a FM, allowing the molar masses to be determined with a deviation of less than 10% (represented by the purple bars and purple line) (Fig. 10).
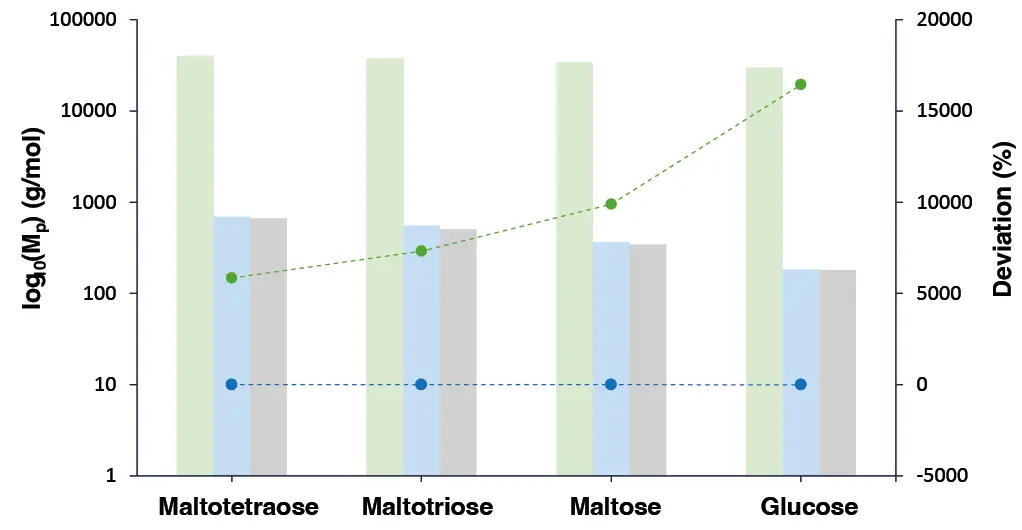
Fig. 10 The molar mass (MP) of four sugars (maltotetraose, maltotriose, maltose and glucose) is plotted on a logarithmic Y-axis (log₁₀) scale. For each compound, three bars are shown: the actual MP (grey), the calculated MP with flow rate correction (pink) and the calculated MP without flow rate correction (green). The dotted lines show the percentage deviation between the calculated and actual MP values. The purple line represents the deviation of the MP values calculated with flow rate correction from the actual MP values, while the green line indicates the deviation of those calculated MP values without flow rate correction.
The molar mass of glucose was determined as an example (Fig. 11). When the flow rate is increased, glucose elutes faster from the column, which is observed in the chromatogram as an earlier peak. Without applying the flow rate correction to adjust the calibration curve (calibration A1), the glucose peak appears further forward within the calibration range of the curve (Fig. 11). This leads to distorted and overestimated molar mass determination (Fig. 10). By adjusting the calibration points using the FM peak according to Eq. 3 and Eq. 4, the calibration curve A1 was corrected (calibration A2). This adjustment ensures that the glucose peak now appears further back within the calibration range of the adjusted calibration curve (Fig. 11), allowing for accurate molar mass determination despite deviation in flow rate (Fig. 10).
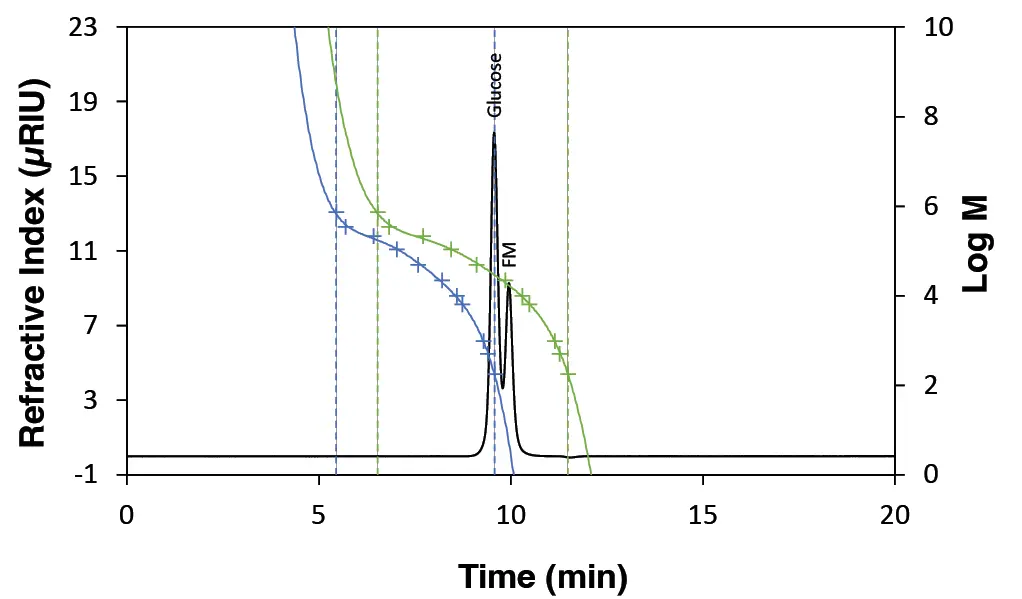
Fig. 11 Chromatogram of glucose with FM. Green: calibration A1 without FR correction; blue: calibration A2 with FR correction.
Conclusion
The series of experiments demonstrated that implementing a FM in GPC/SEC analyses significantly enhances data reproducibility and accuracy. The experiments showed that flow fluctuations during standard measurements can be effectively corrected using the FM, leading to more reliable calibration curves. Furthermore, we confirmed the accuracy of the correction calculations by measuring a standard mixture at various flow rates, demonstrating the ability of the FM to compensate flow rate variations. Finally, the experiments illustrated that deviations in flow rate during sample measurement from those used in calibration can be corrected through FM based correction, ensuring precise molar mass determinations. Overall, the use of a FM is a simple, practical, and effective approach to improve the quality of GPC/SEC data, but attention must be paid to the solubility of the FM in the mobile phase, its detectability and the prevention of interaction or co-elution with other peaks.
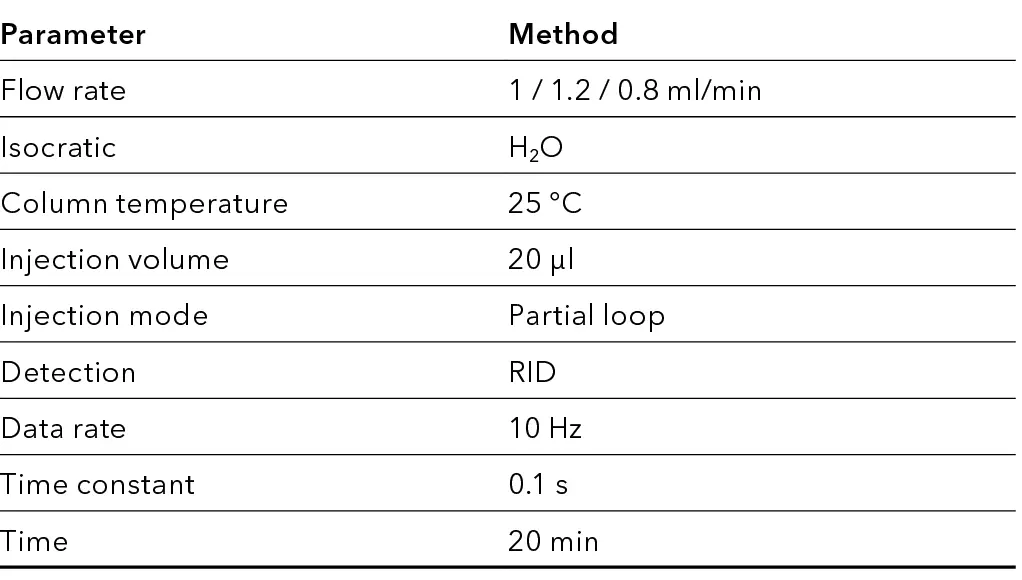
Tab. 3 Method parameter.
Tab. 4 SEC system configuration.
Instrument | Description | Article No. |
Pump | AZURA® P 6.1L Isocratic Pump with 10 ml pump head, stainless steel | |
Autosampler | AZURA® AS 6.1L | |
Detector | AZURA® RID 2.1L | |
Thermostat | AZURA® CT 2.1 | |
Eluent tray | AZURA® E 2.1 L | |
Column | AppliChrom SuperOH-P-Multipore, 7 µm, 300 x 8 mm, 100 - 1 000 000 Da | |
Capillaries | Start-Up Kit with flexible, precut capillaries for analytical HPLC systems with 1/16" connections | |
Software | ClarityChrom® 9.1.0 - Workstation, autosampler control included | |
Software | ClarityChrom® 9.1.0 - SEC/GPC extension |

Fig. 12 SEC system setup; from top to bottom: eluent tray with bottles, pump, refractive index detector (RID), autosampler; right: thermostat with columns.
Application details
|
Method |
GPC/SEC |
|
Mode |
GPC/SEC |
|
Substances |
Ethylene glycol, Pullulan, Maltose, Glucose, Maltotriose, Maltotetraose |
|
CAS number |
107-21-1, 9057-02-7, 6363-53-7, 50-99-7, 1109-28-0, 34612-38-9 |
|
Version |
Application No.: VTN0043 | Version 1 7/2025 | ©KNAUER Wissenschaftliche Geräte GmbH |